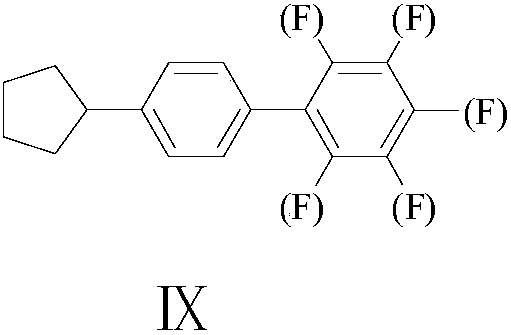
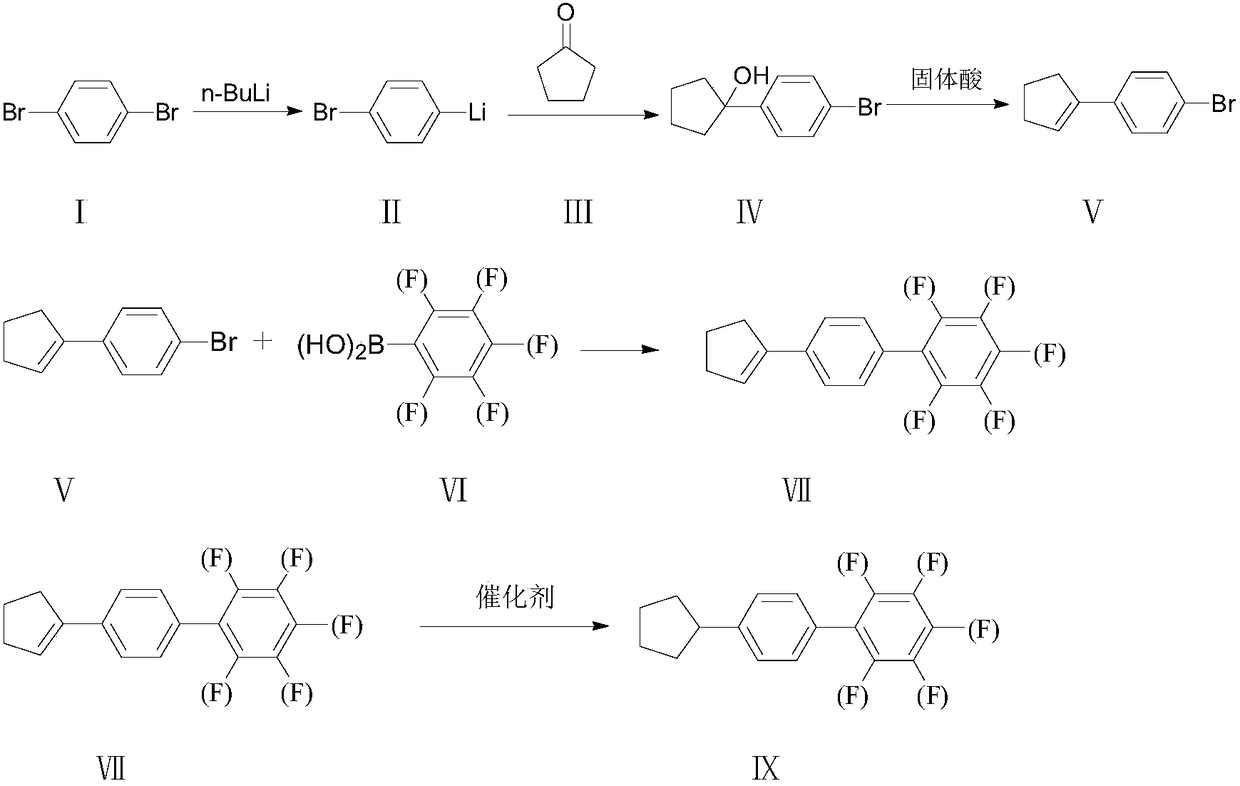
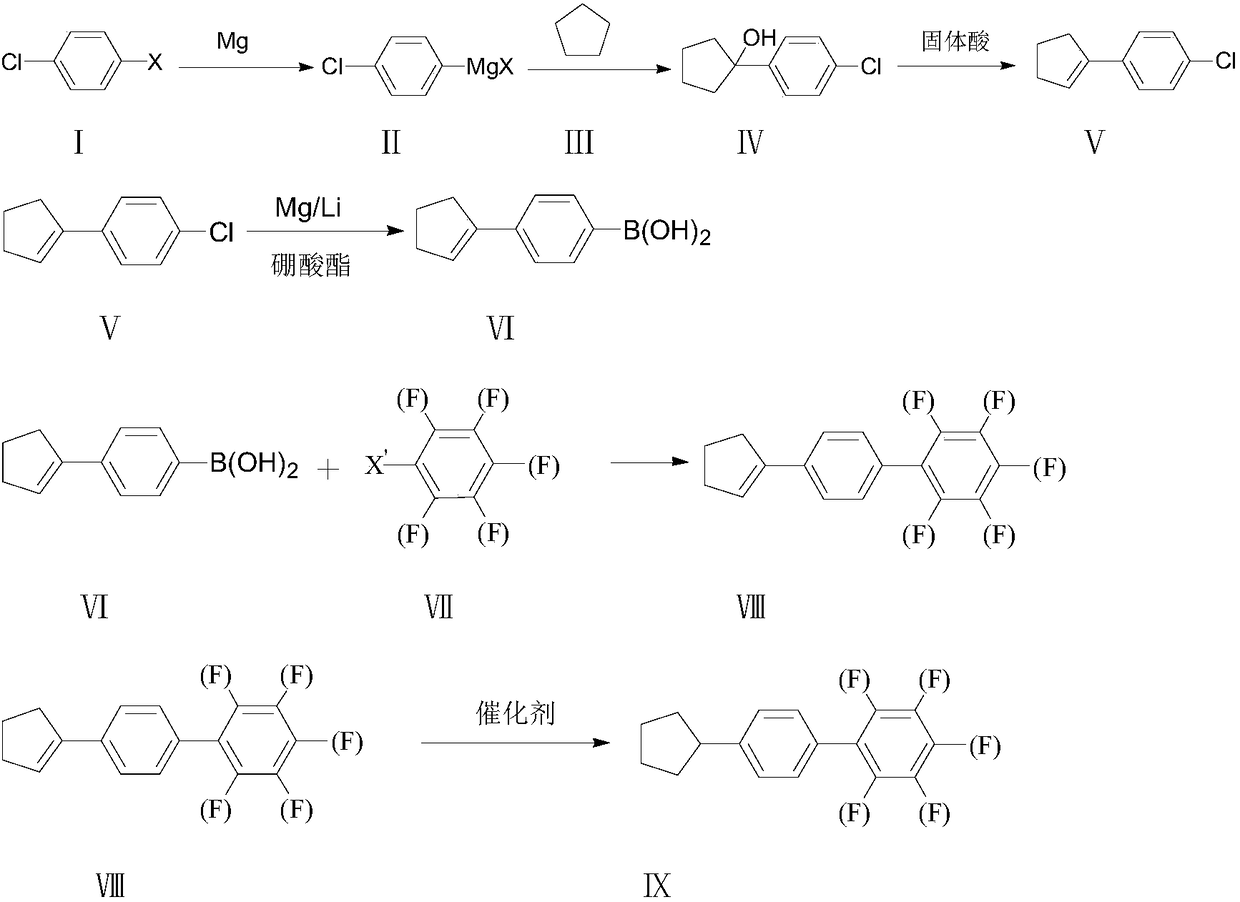
Synthesis method of 4-cyclopentyl biphenyl fluorinated compound
A technology of cyclopentyl biphenyl and cyclopentenyl biphenyl is applied in the field of synthesizing fluorine-containing compounds of 4-cyclopentyl biphenyl, and can solve the problem of low yield of fluorine-containing compound IX, high environmental protection pressure and shortened service life of facilities and other problems, to achieve the effect of convenient post-processing, high safety performance, and low environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1, a synthesis method of 4-cyclopentylbiphenyl fluorine-containing compound IX, this embodiment is a synthesis method of 4'-cyclopentyl-3,5-difluorobiphenyl, the synthesis route is as follows:
[0019]
[0020]
[0021] Wherein the preparation of 4-cyclopentenyl chlorobenzene V is: under the protection of inert gas, in the 500ml reaction bottle that mechanical stirring, reflux condensation drying tube, feeding funnel are housed, add magnesium sheet 13g, THF35ml successively, stir; To 50°C, add dropwise 20ml of the prepared p-chlorobromobenzene 100g / THF100ml mixed solution into the system, the reaction is initiated, and the temperature is controlled at 50°C to 70°C to add dropwise. A good cyclopentanone 50g / toluene 200ml mixed solution, stop the reaction 90min after the dropwise addition, pour the reaction solution into a 500ml reaction bottle with 100ml of concentrated hydrochloric acid, stir for 2h, then add 100ml of toluene, stir for 1h and let it stand ...
Embodiment 2
[0025] Example 2, a synthesis method of 4-cyclopentylbiphenyl fluorine-containing compound IX, this embodiment is a synthesis method of 4'-cyclopentyl-3,4,5-trifluorobiphenyl, the synthesis route is as follows:
[0026]
[0027] Wherein the preparation of 4-cyclopentenyl chlorobenzene V is: under the protection of inert gas, in the 500ml reaction bottle that mechanical stirring, reflux condensation drying tube, feeding funnel are housed, add magnesium sheet 13g, THF35ml successively, stir; To 70°C, add dropwise 20ml of the prepared p-dichlorobenzene 77g / THF100ml mixed solution into the system, the reaction is initiated, and the temperature is controlled at 70°C to 80°C to add dropwise. A good cyclopentanone 50g / toluene 200ml mixed solution, 120min after the dropwise addition, stop the reaction, pour the reaction solution into a 500ml reaction bottle with 100ml of concentrated hydrochloric acid, stir for 2h, then add 100ml of toluene, stir for 1h and let it stand Separate th...
Embodiment 3
[0031] Embodiment 3, a synthetic method of 4-cyclopentylbiphenyl fluorine-containing compound IX, this embodiment is a synthetic method of 4'-cyclopentyl-3-fluorobiphenyl, the synthetic route is as follows:
[0032]
[0033] Wherein the preparation of 4-cyclopentenyl chlorobenzene V is: under the protection of inert gas, in the 500ml reaction bottle that mechanical stirring, reflux condensation drying tube, feeding funnel are housed, add magnesium sheet 13g, THF35ml successively, stir; To 70°C, add dropwise 20ml of the prepared p-chloroiodobenzene 125g / THF100ml mixed solution into the system, the reaction is initiated, and the temperature is controlled at 40°C to 50°C to add dropwise. A good cyclopentanone 50g / toluene 200ml mixed solution, 120min after the dropwise addition, stop the reaction, pour the reaction solution into a 500ml reaction bottle with 100ml of concentrated hydrochloric acid, stir for 2h, then add 100ml of toluene, stir for 1h and let it stand Separate the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com