Enhanced wearable therapy device paired with insertable cardiac monitor
A technology of treatment device and monitoring device, applied in the direction of cardiac defibrillator, treatment, sensor, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
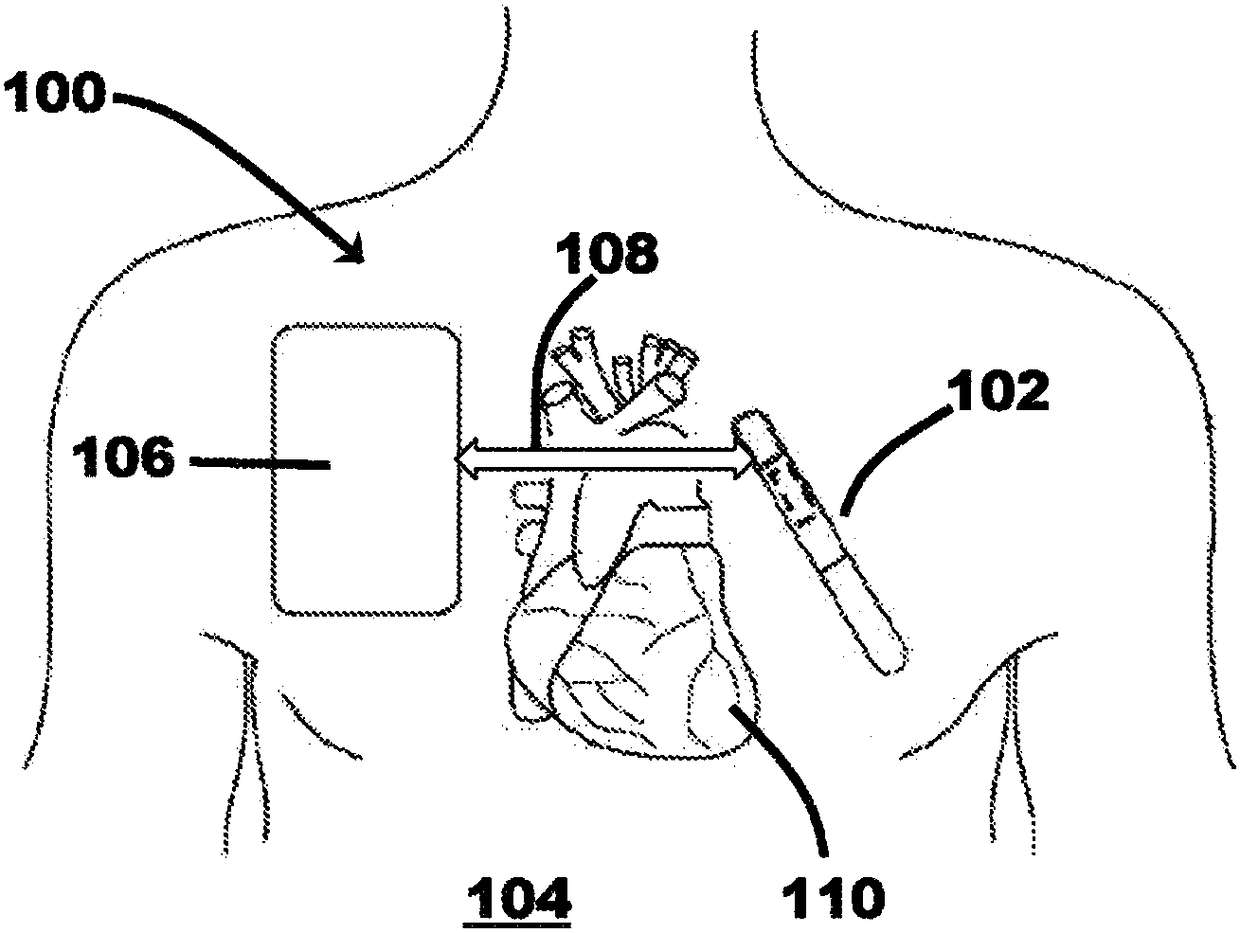
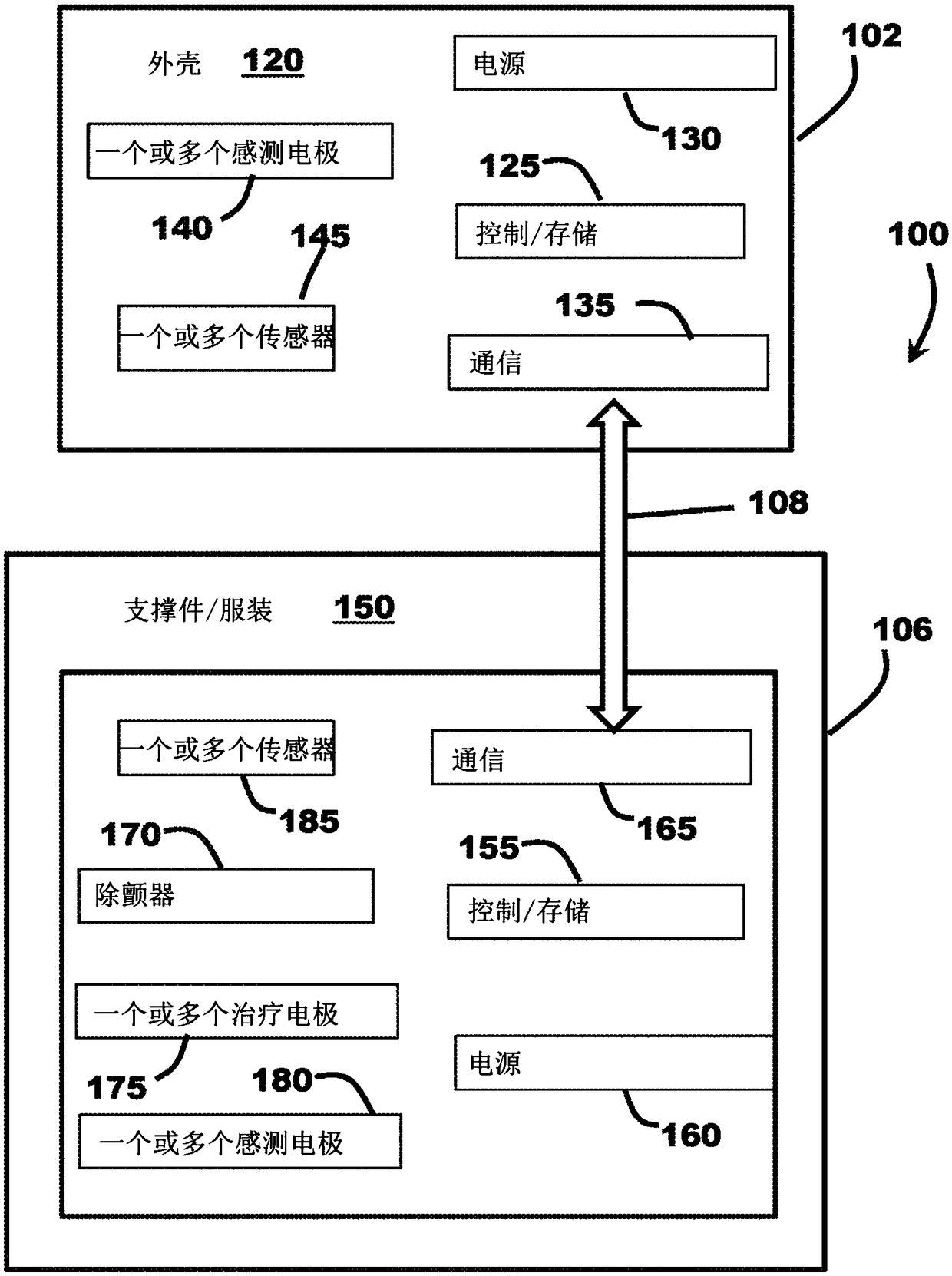
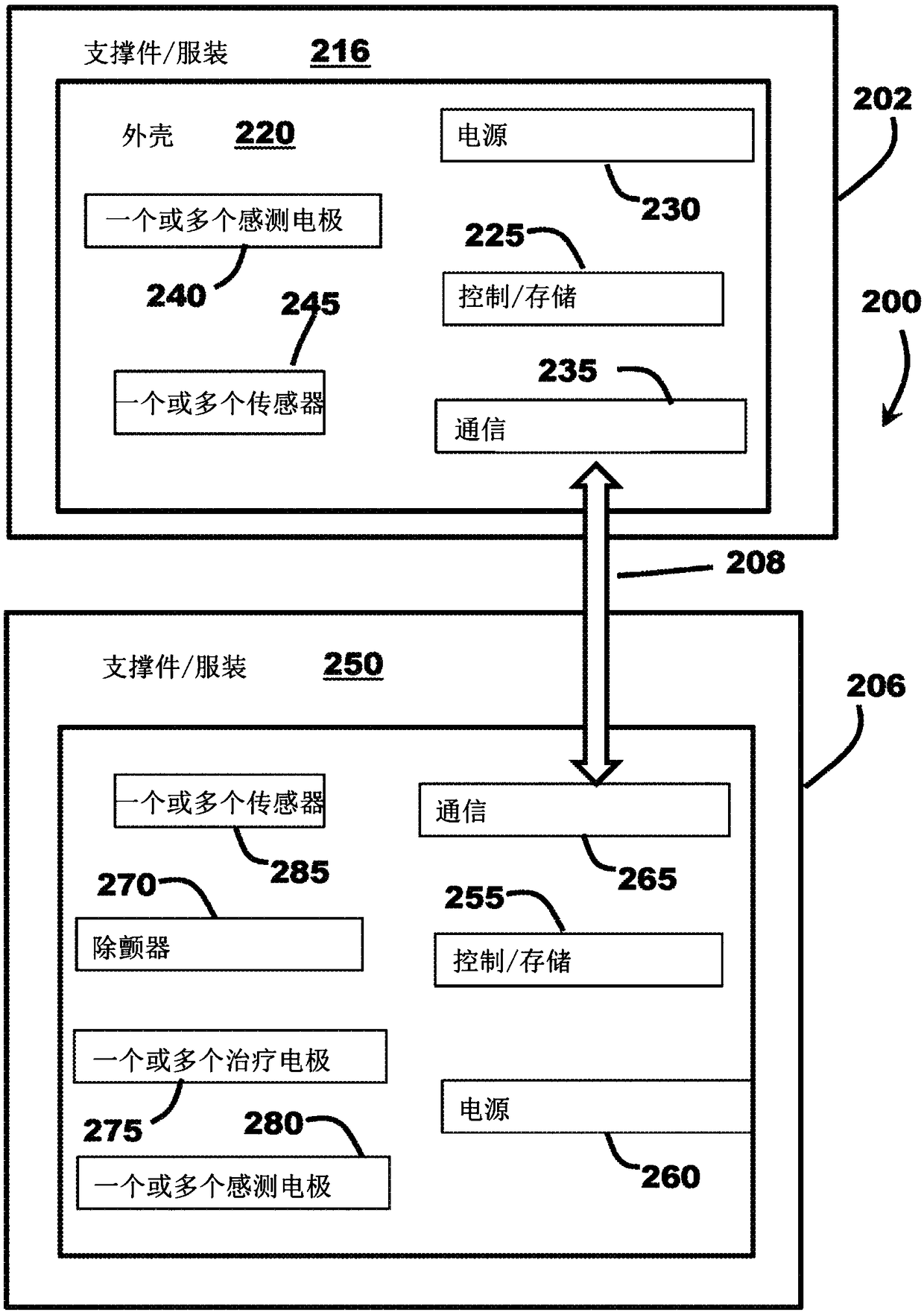
[0046] figure 1 is a schematic diagram of a medical system 100 including a cardiac monitoring device (CMD) 102 and an external therapeutic device 106 communicatively coupled to the CMD 102 via a communication link 108 . In the illustrated embodiment, medical system 100 is operably coupled to patient 104 , and CMD 102 and treatment device 106 are configured to communicate with each other via communication link 108 . Patient 104 can be a human, dog, pig, and / or any other animal that has physiological parameters that can be recorded. In various embodiments, as explained in further detail below, the CMD 102 may be implanted subcutaneously in an implant site or pocket in the patient's chest or abdomen, while in other embodiments, the CMD 102 may be configured to be A wearable device worn at 104 , eg, integrated into a vest, belt, harness, and / or sticker, etc., and may be configured to monitor (eg, sense and / or record) physiological parameters associated with the patient's heart 11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com