Method and device for indicating acute myelogenous leukemia medicine
A technology for acute myeloid and leukemia, applied in biochemical equipment and methods, microbial measurement/inspection, proteomics, etc., can solve the problems of cumbersome gene screening process, inability to formulate treatment plan, delay in treatment timing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
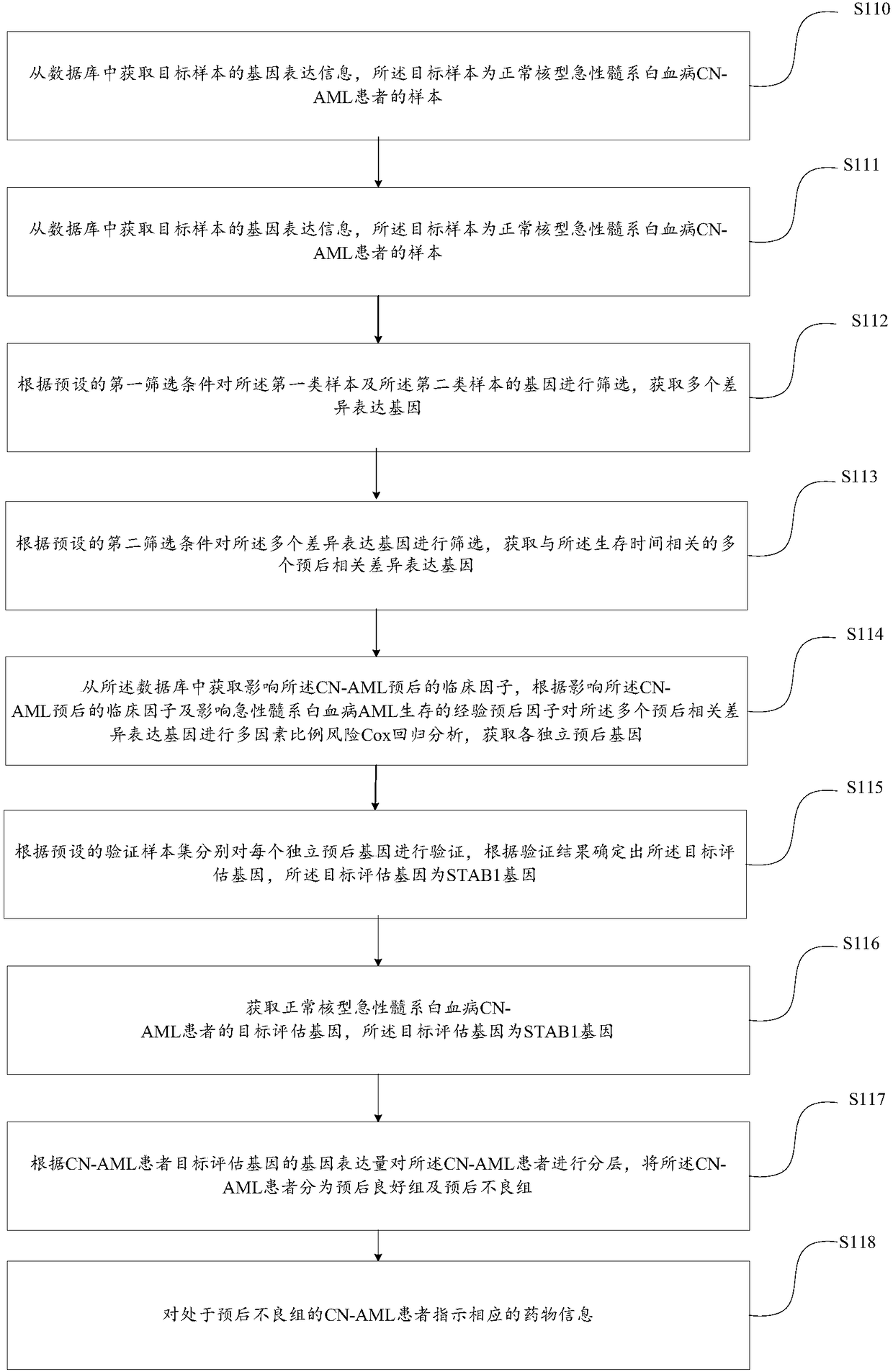
[0073] This embodiment provides a method for drug indication for acute myeloid leukemia, such as figure 1 As shown, the method includes:
[0074] S110, acquiring gene expression information of a target sample from a database, where the target sample is a sample of a CN-AML patient with normal karyotype acute myeloid leukemia;
[0075] In this step, the gene expression information of a preset number of samples is downloaded from the cancer gene database (TCGA, The Cancer Genome Atlas). The preset samples include: patients with normal karyotype acute myeloid leukemia CN-AML and abnormal karyotype acute myeloid leukemia. The gene expression information of leukemia patients, the gene expression information includes: gene expression amount.
[0076] Here, because in the data, the sample identifications of CN-AML patients and patients with abnormal karyotype acute myeloid leukemia are different, so the gene of the target sample can be extracted from the preset number of samples acc...
Embodiment 2
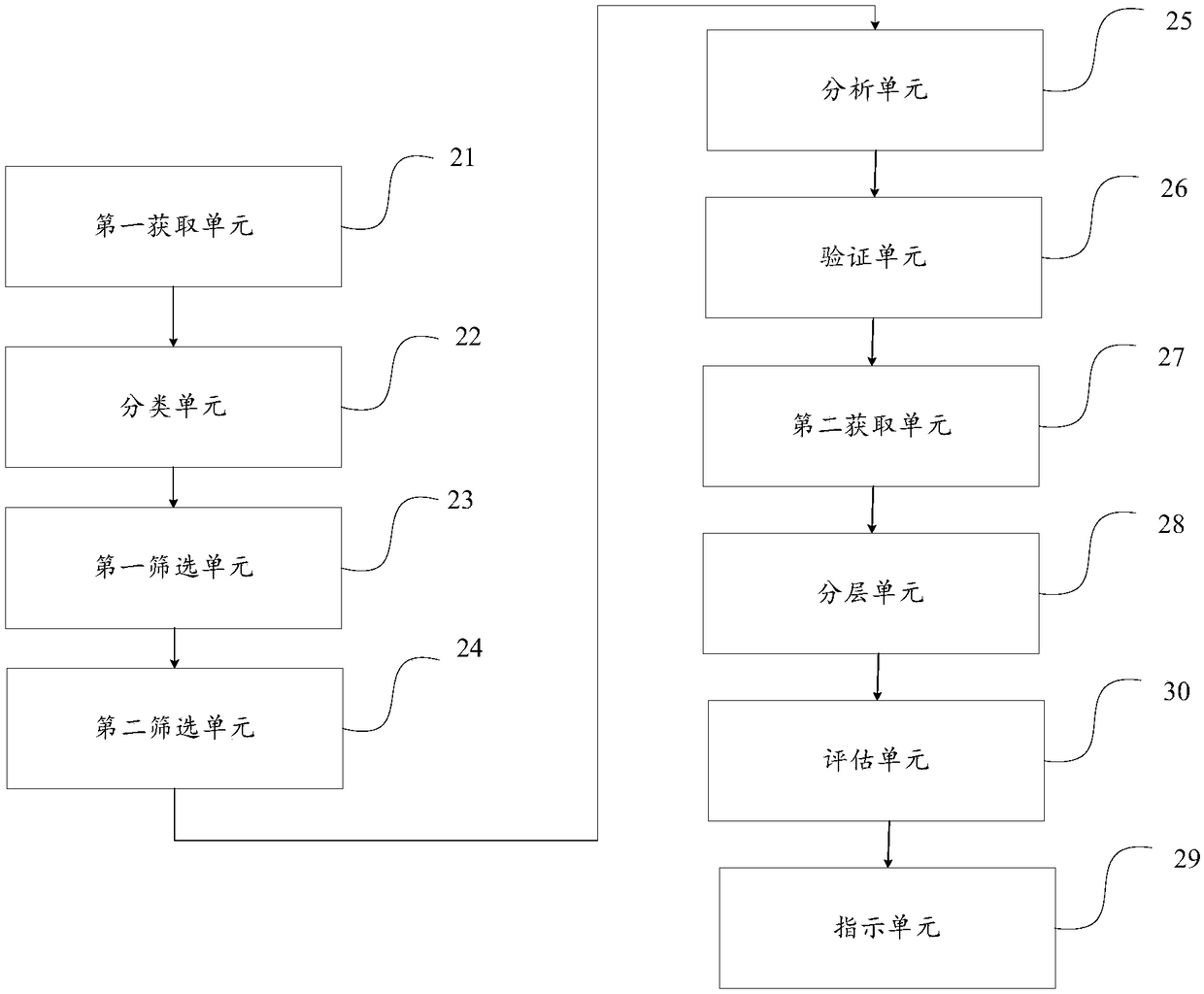
[0132] Corresponding to Embodiment 1, this embodiment provides a device for acute myeloid leukemia drug indication, such as figure 2 As shown, the device includes: a first acquisition unit 21, a classification unit 22, a first screening unit 23, a second screening unit 24, an analysis unit 25, a verification unit 26, a second acquisition unit 27, a stratification unit 28 and an indication Unit 29; where,
[0133] Before the first acquisition unit 21 acquires the target evaluation gene of the patient with normal karyotype acute myeloid leukemia CN-AML, it needs to first determine which gene the target evaluation gene is, and the specific determination method is as follows:
[0134] The first acquisition unit 21 is used to download and acquire gene expression information of a preset number of samples from the cancer gene database TCGA, and the preset samples include: patients with normal karyotype acute myeloid leukemia CN-AML and patients with abnormal karyotype acute myeloid ...
Embodiment 3
[0171] In practical applications, the target evaluation gene of CN-AML can be determined according to the above method and device, and the gene can be used to stratify the prognosis of CN-AML and indicate the corresponding drug information, as follows:
[0172] First download the gene expression information and clinical information of 200 samples from the TCGA database, and then extract the gene expression information of the CN-AML samples from the preset number of samples according to the sample identification of the CN-AML samples. The number of CN-AML samples is 79 cases.
[0173] According to the preset survival time as the classification standard, the CN-AML samples are divided into the first type of samples and the second type of samples; the preset survival time is an empirical index of clinical complete remission of CN-AML, specifically: 2 years. In this implementation, the first type of samples are CN-AML samples whose survival time is less than 2 years, and the seco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com