Preparation of functionalized carbon material based on Schiff base-MOF and application of functionalized carbon material as electrocatalyst
A technology of -MOF and Schiff base, applied in the field of preparation of nitrogen-functionalized carbon materials, can solve the problems of ORR and OER catalytic performance degradation, high raw material prices, poor catalyst stability, etc., and achieve excellent methanol tolerance, preparation method Simple, long-lasting results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
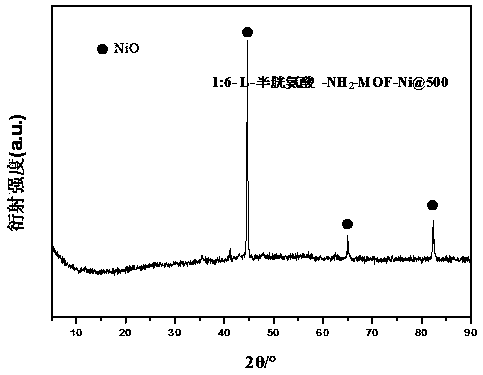
[0041] (1) NH 2 - Preparation of MOF-Ni: Take 1.81g (0.01mol) of 2-aminoterephthalic acid and NiCl 2 ·6H 2 O 14.26g (0.06mol), added to 50ml DMF and mixed into a uniform and stable solution; then the mixed solution was placed in a hydrothermal box, and reacted hydrothermally at 200°C for 18 hours, and the obtained product was washed 6 times with DMF, centrifuged, Vacuum drying at 60°C for 12 hours to obtain the product NH 2 -MOF-Ni;
[0042] (2) Preparation of Schiff base-MOF precursor: Weigh NH 2 -MOF-Ni 0.2g and L-cysteine (L-Cysteine) 1.2g, (NH 2 -MOF-Ni and L-cysteine mass ratio is 1:6), respectively added to 30mlCH 2 Cl 2 in, mix and stir, stir and heat to reflux at 60°C for 6h, centrifuge, and use CH 2 Cl 2 Wash 3~5 times, dry in vacuum at 60°C for 24 hours to obtain the precursor L-cysteine-NH 2 -MOF-Ni;
[0043] (3) Preparation of nitrogen-doped functionalized carbon materials: the precursor L-cysteine-NH 2 -MOF-Ni is carbonized at a high temperature of ...
Embodiment 2
[0046] (1) NH 2 - Preparation of MOF-Ni: Take 1.81g (0.01mol) of 2-aminoterephthalic acid and FeCl 3 ·6H 2 O 16.21g (0.06mol), added to 50ml DMF and mixed into a uniform stable solution; then the mixed solution was placed in a hydrothermal box, and hydrothermally reacted at 200°C for 18 hours, and the obtained product was washed 6 times with DMF, centrifuged, Vacuum drying at 60°C for 12 hours to obtain the product NH 2 -MOF-Fe;
[0047] (2) Preparation of Schiff base-MOF precursor: Weigh NH 2 -MOF-Fe 0.2g and L-cysteine (L-Cysteine) 1.2g, (NH 2 -MOF-Fe and L-cysteine mass ratio is 1:6), respectively added to 30mlCH 2 Cl 2 in, mix and stir, stir and heat to reflux at 60°C for 6h, centrifuge, and use CH 2 Cl 2 Wash 3~5 times, dry in vacuum at 60°C for 24h to obtain the precursor L-cysteine-NH 2 -MOF-Fe;
[0048] (3) Preparation of nitrogen-doped functionalized carbon materials: the precursor L-cysteine-NH 2 -MOF-Fe was carbonized at 500°C for 3 hours in a nitroge...
Embodiment 3
[0051] (1) NH 2 - Preparation of MOF-Ni: Take 1.81g (0.01mol) of 2-aminoterephthalic acid and NiCl 2 ·6H 2 O 14.26g (0.06mol), added to 50ml DMF and mixed into a uniform stable solution; then the mixed solution was placed in a hydrothermal box, and hydrothermally reacted at 200°C for 18 hours, and the obtained product was washed 6 times with DMF, centrifuged, Vacuum drying at 60°C for 12 hours to obtain the product NH 2 -MOF-Ni;
[0052] (2) Preparation of Schiff base-MOF precursor: Weigh NH 2 -MOF-Ni 0.2g and L-Lysine (L-Lysine) 1.2g, (NH 2 -MOF-Ni and L-lysine mass ratio is 1:6), respectively added to 30mlCH 2 Cl 2 in, mix and stir, stir and heat to reflux at 60°C for 6h, centrifuge, and use CH 2 Cl 2 Wash 3~5 times, dry in vacuum at 60°C for 24 hours to obtain the precursor L-lysine-NH 2 -MOF-Ni;
[0053] (3) Preparation of nitrogen-doped functionalized carbon materials: the precursor L-lysine-NH 2 -MOF-Ni is carbonized at a high temperature of 500°C for 3 hours ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com