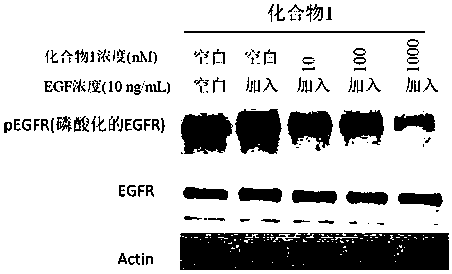
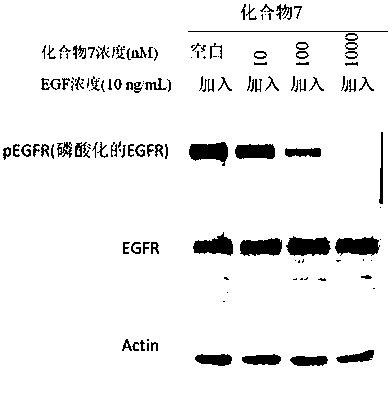
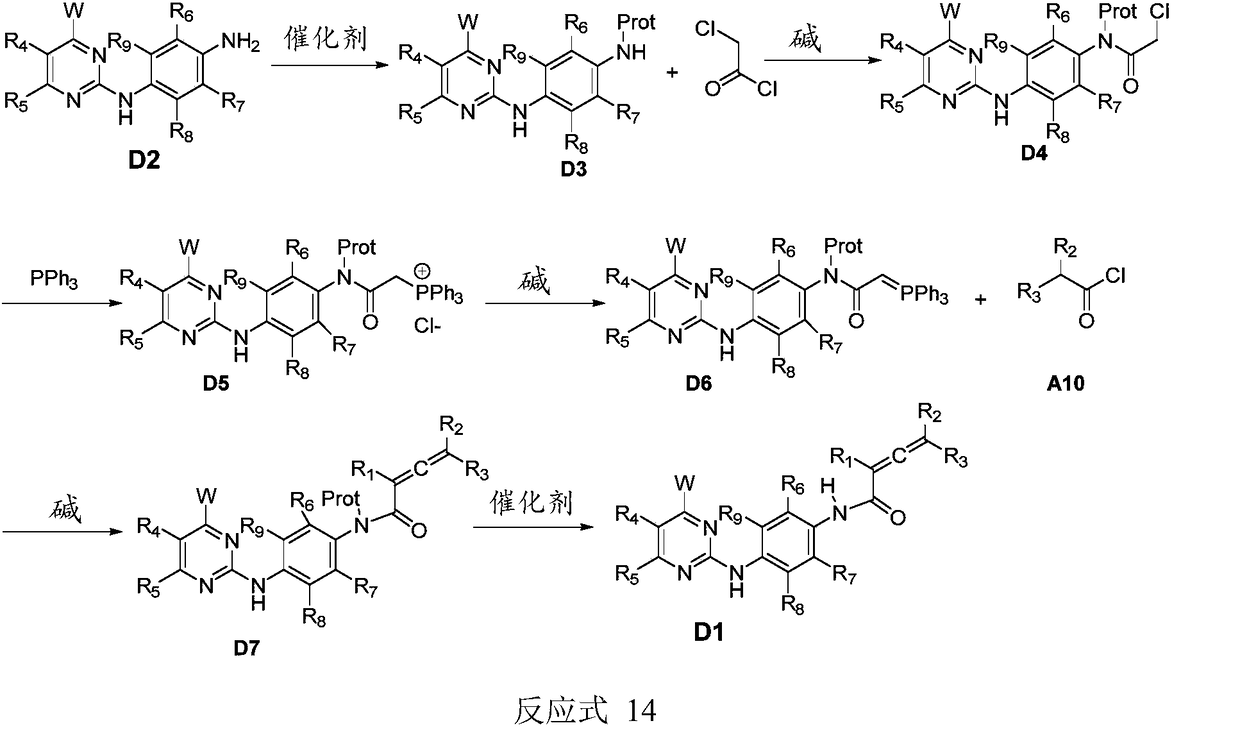
Compound containing conjugated allene amide structure and preparation method, medicine composition and application thereof
A technology for compounds and alkyl groups, which is applied in the fields of compounds containing conjugated allenamide structures, their preparation, pharmaceutical compositions and uses, and can solve problems such as uncertainties in the biological activity and pharmaceutical properties of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0180] Example 1 Synthesis of N-[2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(1-methyl-1H-indyl) (Dole-3-yl)pyrimidin-2-yl)amino)phenyl)-2,3-butenamide (1)
[0181]
[0182] Step 1: Synthesis of 3-(2-chloropyrimidin-4-yl)-1H-indole (GB011)
[0183] Under 0℃ and nitrogen protection, the CH 3 MgCl (3M, tetrahydrofuran solution, 14 mL, 42 mmol) was added dropwise to a solution of indole (5 g, 42 mmol) in 1,2-dichloroethane (250 mL). The resulting solution was stirred for 15 minutes, and then 2,4-dichloropyrimidine (9.48 g, 64 mmoL) was added all at once. The resulting solution was allowed to warm to room temperature and stirred for an additional 16 hours. The reaction was quenched by adding methanol (25 mL), and then the resulting mixture was concentrated in vacuo, the residue was adsorbed on silica gel, and purified by a normal phase silica gel column to obtain GB011 (3 g) as a yellow-like solid. 1 H NMR(DMSO-d 6 ,400MHz): 7.18-7.27(m,2H),7.46-7.53(m,1H), 7.90(d,1H,J=...
Embodiment 2
[0198] Example 2 Synthesis of (S)-N-[2-(tetrahydrofuran-3-yl)oxy-4-methoxy-5-((4-(1-methyl-1H-indol-3-yl) (Pyrimidine-2-yl)amino)phenyl)-2,3-butenamide (2)
[0199]
[0200] Step 1: Synthesis of (S)-N-(2-methoxy-4-(tetrahydrofuran-3-yl)oxy-5-nitro)phenyl-4-(1-methyl-1H-indole- 3-yl)pyrimidin-2-ylamine (GB056)
[0201] Add potassium tert-butoxide (0.366g, 3.81mmol) to N,N-dimethylformamide (10mL) and stir at 0℃, add 0.167g (1.9mmol) of (S)-(+)-3-hydroxyl Tetrahydrofuran, react for 1 hour. Add GB17 (0.500g, 1.27mmol) and react at room temperature for 3 hours. Add 100 mL of water to the obtained suspension, adjust the pH to 7-8, stir, filter with suction, dry the filter cake, and purify by silica gel column to obtain 0.15 g of the target GB056. 1 H NMR(DMSO-d 6 ,400MHz): 8.77 (s, 1H), 8.28-8.40 (m, 3H), 8.18 (s, 1H), 7.51 (d, 1H, J = 7.95 Hz), 7.20-7.28 (m, 2H), 7.07- 7.13(m,1H),6.93(s,1H),5.34-5.41 (m,1H),4.00(s,3H),3.93-3.98(m,1H),3.76-3.92(m,2H),3.87( s, 3H), 3.76-3.84 (m, 1H)...
Embodiment 3
[0206] Example 3 Synthesis of N-[2-(4-methylpiperazinyl)-4-methoxy-5-((4-(1-methyl-1H-indol-3-yl)pyrimidine-2- (Yl)amino)phenyl)-2,3-butenamide (3)
[0207]
[0208] Step 1: Synthesis of N-(4-(4-methylpiperazinyl)-2-methoxy-5-nitrophenyl)-4-(1-methyl-1H-indol-3-yl) Pyrimidine-2-ylamine (GB066)
[0209] Add GB017 (0.1g, 0.254mmol) into 5mL N-pyrrolidone and stir at room temperature, add nitrogen methyl piperazine (0.025g, 0.254mmol), then add diisopropylethylamine (0.1mL), and heat to 140°C reaction. React for 2.5 hours and cool to room temperature. 100 mL of water was added to the above reaction solution, filtered with suction, the filter cake was dried, and purified by a silica gel column to obtain 0.1 g of the target GB066. 1 H NMR(CDCl 3 ,400MHz):9.66(s,1H),8.40(d,1H,J=5.25Hz),8.29(s,1H),8.14-8.20 (m,1H),7.57(s,1H),7.39-7.44( m, 1H), 7.28-7.36 (m, 2H), 7.20 (d, 1H, J = 5.29 Hz), 6.63 (s, 1H), 4.00 (s, 3H), 3.95 (s, 3H), 3.10-3.19 (m, 4H), 2.62-2.72 (m, 4H), 2.40 (s, 3H). E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com