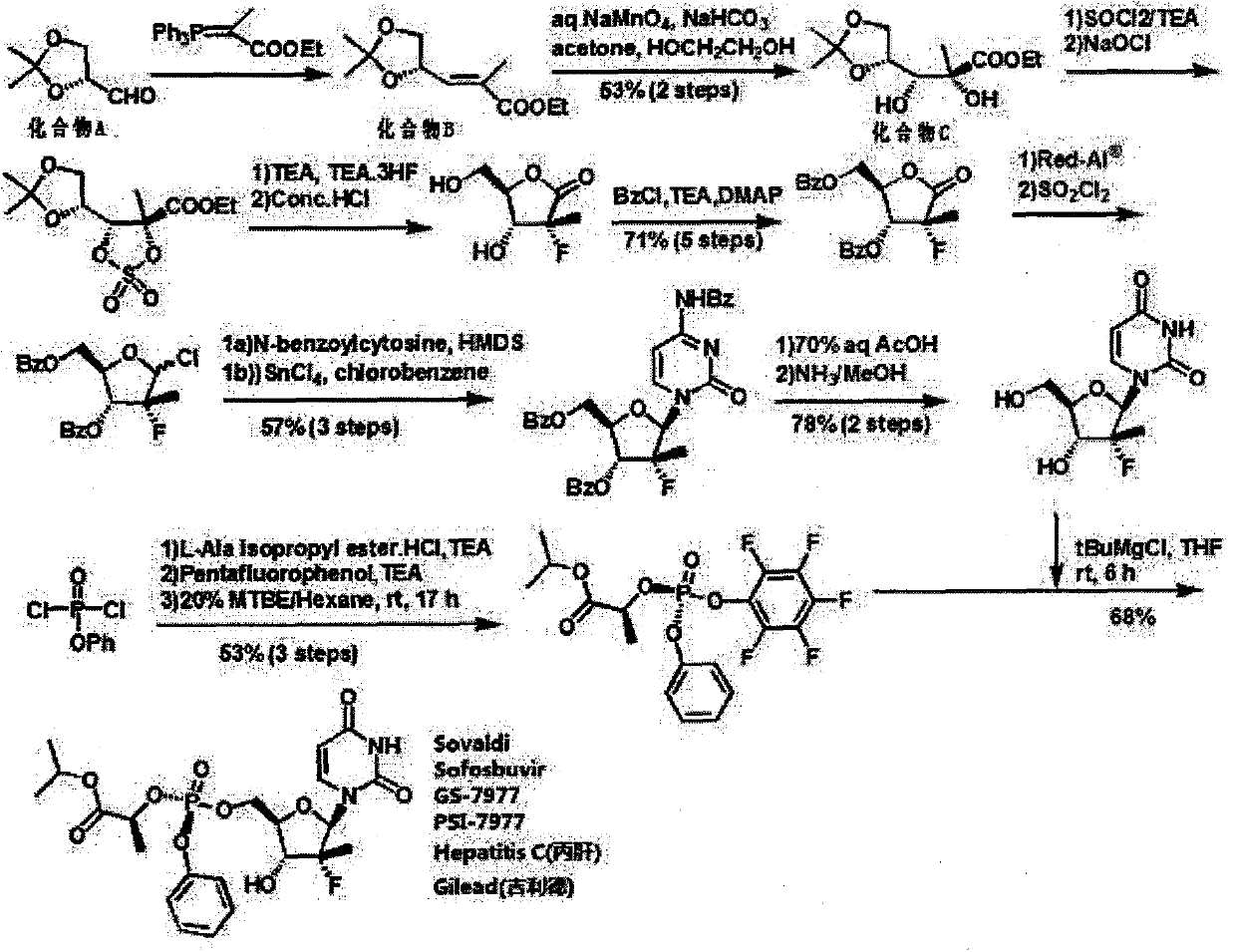
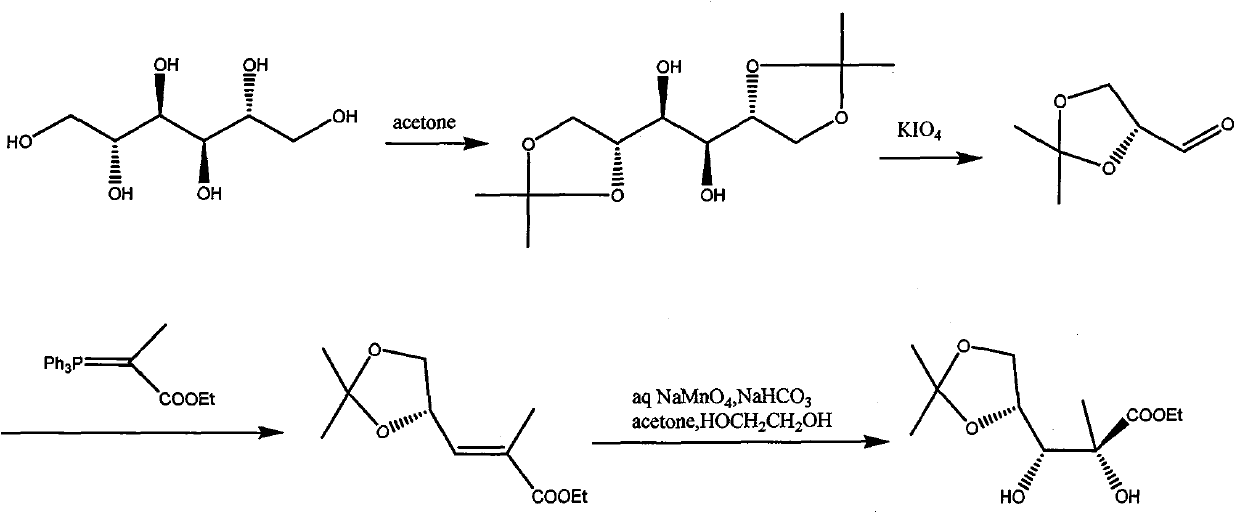
A kind of synthetic method of Sofosbuvir intermediate
A synthetic method and intermediate technology, applied in the field of drug synthesis, can solve the problems of high price of downstream raw materials, impact on the purity and quality of compound C, insufficient purity of compound B, etc., and achieve the prospect of large-scale industrial production, which is conducive to stable production , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the synthesis of intermediate product (III)
[0029] Add 300mL of acetone and 0.3mL of boron trifluoride diethyl ether into a clean 500mL three-necked bottle, stir, cool down to 0°C, and slowly add 18.5g (R)-epichlorohydrin to it dropwise, and the dropwise addition is completed within 30 minutes. After 13 hours, the reaction was stopped, the acetone was distilled off, and 24 g of pure product was obtained by distillation under reduced pressure, with a yield of 80%.
Embodiment 2
[0030] Embodiment 2: the synthesis of intermediate product (IV)
[0031] Add 200mL triethyl phosphite into a clean 500mL three-neck flask, add 24g of intermediate product (III) to it, heat to 65°C, reflux for 24h, stop the reaction, remove excess triethyl phosphite by distillation, and distill the three-necked The remaining liquid in the bottle was purified by a silica gel column to obtain 36.2 g of the product with a yield of 89.2%.
Embodiment 3
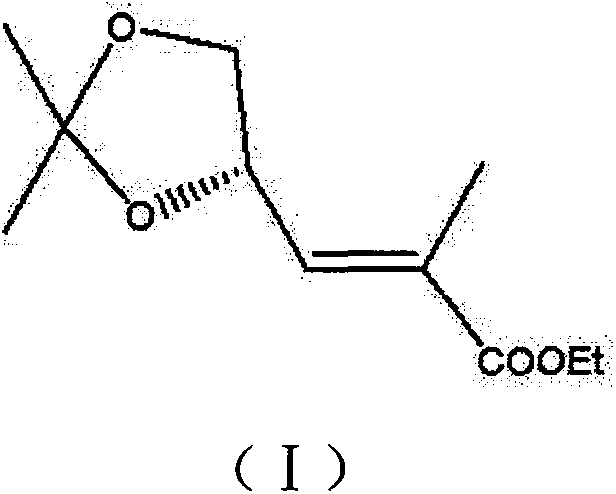
[0032] Embodiment 3: the synthesis of intermediate (I)
[0033] Add the intermediate product (IV) to 500mL of toluene, cool down to 0°C, add 18g of potassium tert-butoxide in batches, stir for 30min, add 17g of ethyl pyruvate dropwise, control the reaction temperature at 0-5°C, and finish the dropwise Complete, react for 12 hours, stop the reaction, lower the temperature to 5°C, add 2N hydrochloric acid dropwise to adjust the pH to 6-7, add 300mL of water, stir and separate layers, wash the organic layer with water, dry, concentrate, and distill to obtain the pure intermediate product (I) 24g, yield 79.1%, purity 99.82%. 1H-NMR (400Hz, CDCl3), δ=6.66(dd, J=6.8, 8.0Hz, 1H), 4.81-4.86(m, 1H), 4.11-4.21(m, 3H), 3.60(t, J=8.4 Hz, 1H), 1.87 (d, J = 1.2 Hz, 3H), 1.43 (s, 3H), 1.38 (s, 3H), 1.27 (t, J = 6.8 Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com