A fluorescent material with piezochromic and lyochromic properties
A fluorescent material and piezochromic technology, applied in the field of fluorescent materials, can solve problems such as limited types and quantities, imperfect color change mechanism, and difficulties in designing and synthesizing such molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
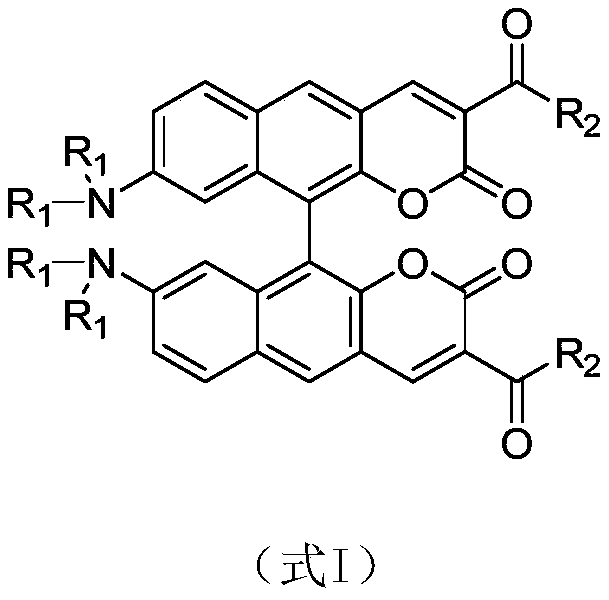
[0021] Example 1: Preparation of 8,8'-bisdimethylamino-10,10'-biphenylcoumarin-3,3'-dicarboxylic acid diethyl ester
[0022] a. Synthesis of intermediate 2,2'-bismethoxymethoxy-7,7'-bisdimethylamino-1,1'-binaphthyl
[0023]
[0024] Under ice-cooling, add 1.26g of sodium hydride (31.5mmol) into 10mL of DMF, then add 2.01g of 7,7'-bisdimethylaminobinaphthol (5.4mmol) to the solution, stir for about 10 minutes, and then 1.73 g of chloromethyl methyl ether (17.0 mmol) was added dropwise to the reaction solution, and the stirring was continued for 1 hour, then the ice-water bath was removed, and then the reaction was stirred at room temperature for 3 hours. After the reaction was complete, ethyl acetate (20 mL) and water (50 mL) were added to the reaction solution, and the organic phase was separated. The aqueous phase (2×15 mL) was extracted with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, spin-dried, and vacuum-dried to obt...
Embodiment 2
[0034] Example 2: The piezochromic ability of target 8,8'-bisdimethylamino-10,10'-biphenylcoumarin-3,3'-dicarboxylate diethyl ester
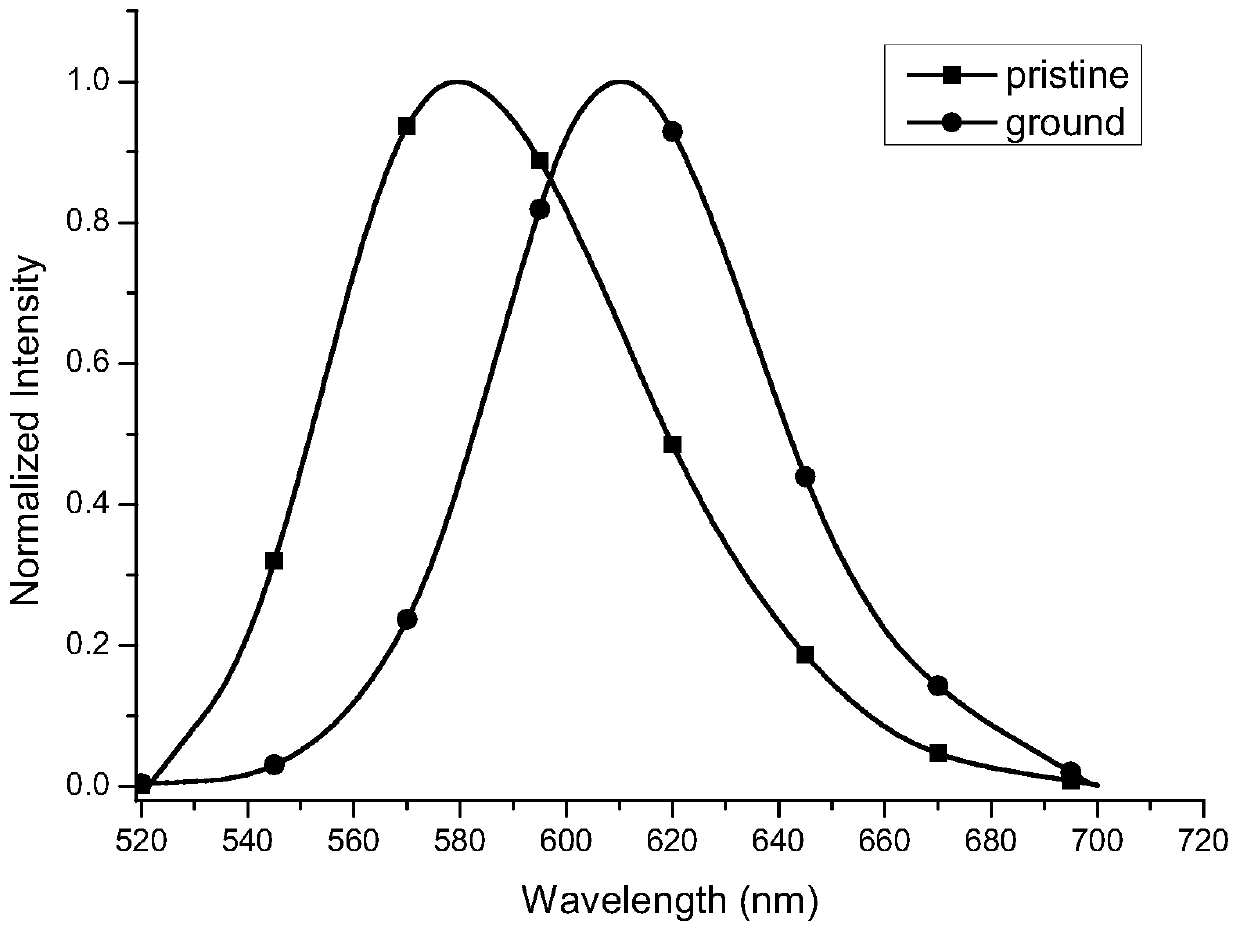
[0035] The compound 8,8'-bisdimethylamino-10,10'-biphenylcoumarin-3,3'-dicarboxylate diethyl ester exhibits orange-yellow fluorescence in the initial state, and it shows Fluorescent red. figure 2 It clearly shows that the solid fluorescence emission wavelength of the compound 8,8'-bisdimethylamino-10,10'-biphenylcoumarin-3,3'-dicarboxylate red-shifted from 579 nm to 610 nm, the wavelength red shift reaches 31 nm. The ground sample was fumigated with dichloromethane gas, and its solid fluorescence returned to the original orange-yellow fluorescence (emission wavelength 579 nm). This shows that such compounds have typical piezochromic properties.
Embodiment 3
[0036] Example 3: Lyochromic properties of target 8,8'-bisdimethylamino-10,10'-biphenylcoumarin-3,3'-dicarboxylate diethyl ester
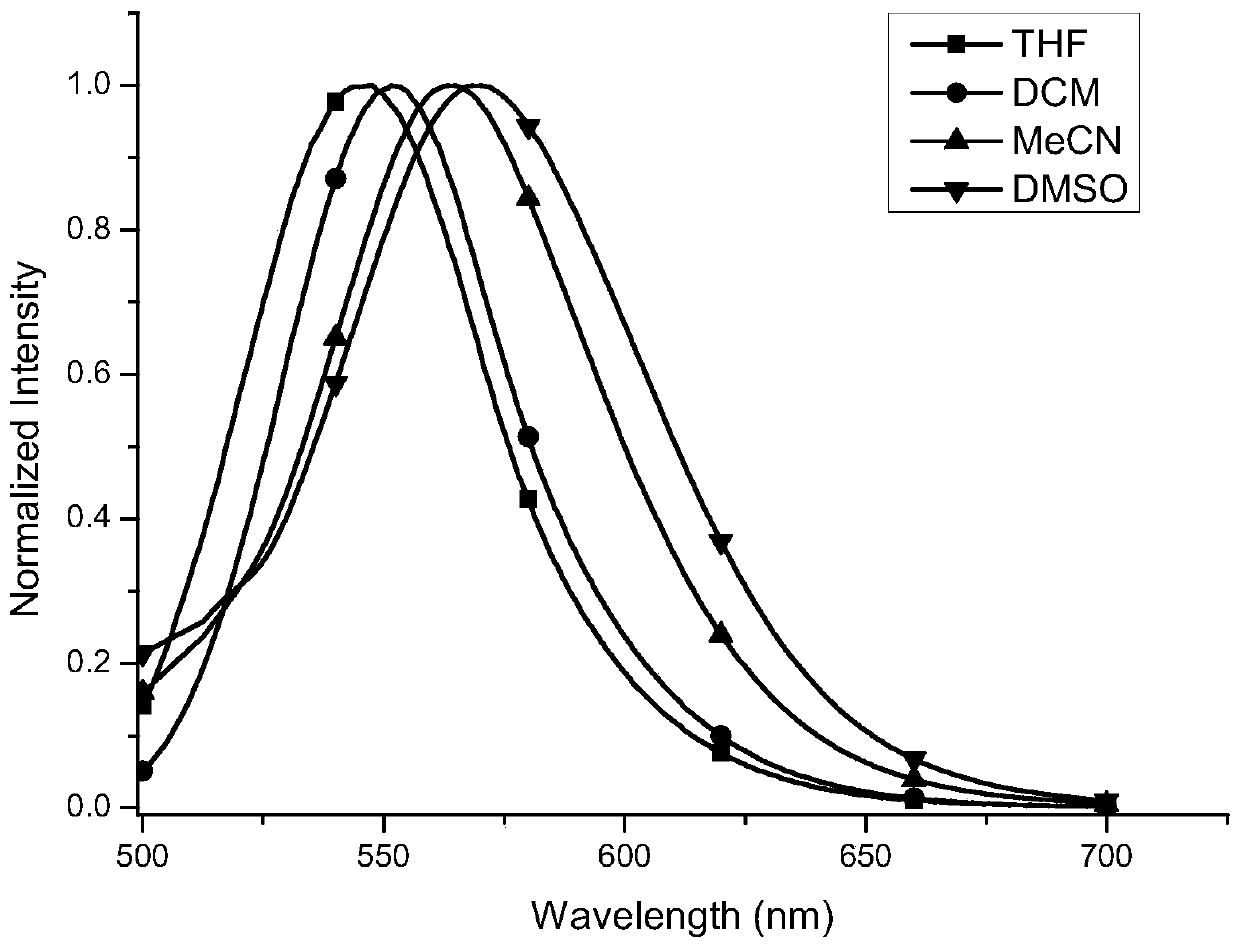
[0037]Compound 8,8'-bisdimethylamino-10,10'-bibenzocoumarin-3,3'-dicarboxylated diethyl The ester concentration is 10 -5 mol / L of the solution to be tested. The fluorescent properties of the above solutions were tested with a Hitachi-F4500 fluorescence spectrophotometer, and the excitation wavelength was 440 nm. Such as figure 1 As shown, the maximum fluorescence emission wavelength of the compound 8,8'-bisdimethylamino-10,10'-biphenylcoumarin-3,3'-dicarboxylate in tetrahydrofuran solvent is 547 nm , 552 nm in dichloromethane, 564 nm in acetonitrile, and 569 nm in dimethylsulfoxide. The above data clearly show that the maximum fluorescence emission peak of the compound 8,8'-bisdimethylamino-10,10'-biphenylcoumarin-3,3'-dicarboxylate diethyl ester increases with the polarity of the solvent. Large gradual redshift. This shows that such compound...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com