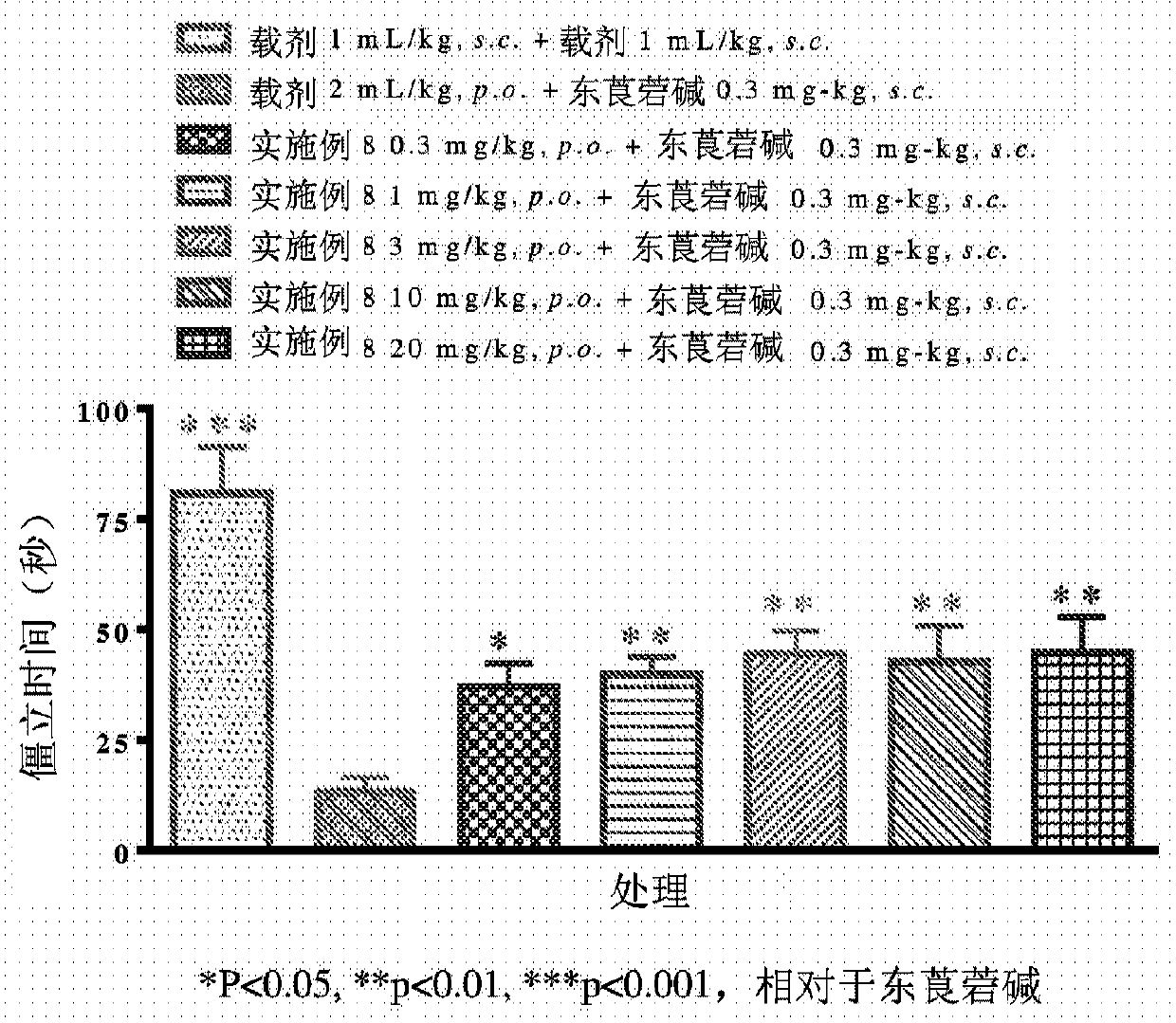
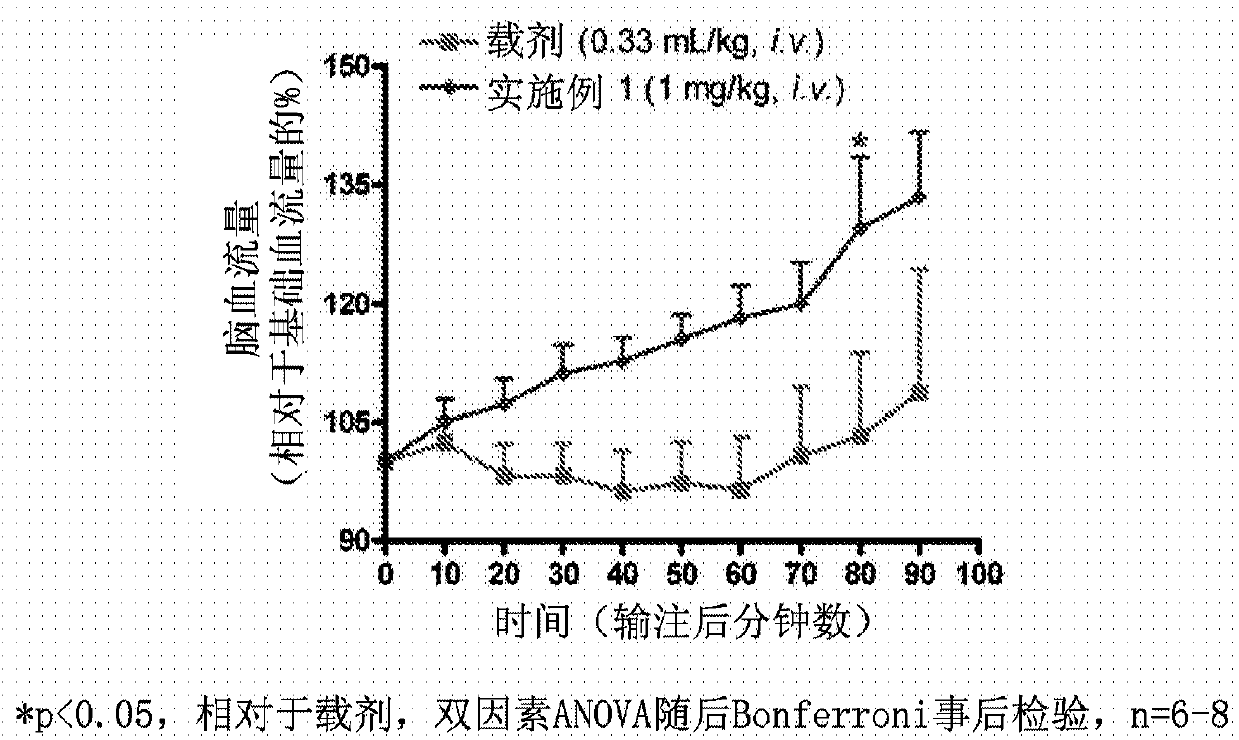
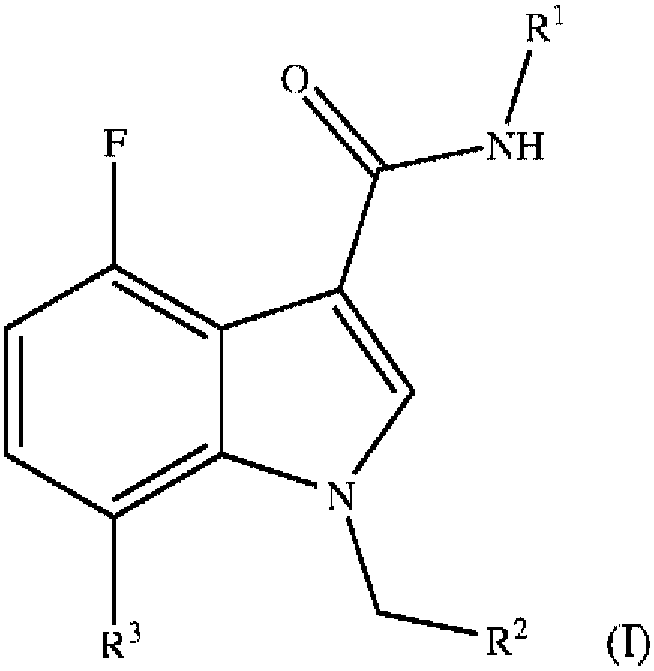
Fluoroindole derivatives as muscarinic m1 receptor positive allosteric modulators
An indole compound, indole technology, applied in the direction of drug combination, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of poor brain permeability and free fraction availability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0330] N-[(1S,2S)-2-Hydroxycyclohexyl]-1-(1-methyl-1H-pyrazol-4-ylmethyl)-4-fluoro-1H-indole-3-carboxamide
[0331]
[0332] Step 1: 1-[1-(1-Methyl-1H-pyrazol-4-ylmethyl)-4-fluoro-1H-indol-3-yl]-2,2,2-trifluoroethanone
[0333]
[0334] in N 2 To a cooled solution of 2,2,2-trifluoro-1-(4-fluoro-1H-indol-3-yl)-ethanone (1-10, 13.30 g, 0.057 mol) in DMF (100 mL) was added K 2 CO 3 (47.06 g, 0.34 mol), 4-chloromethyl-1-methyl-1 H-pyrazole hydrochloride (13.07 g, 0.078 mol) and the contents were stirred overnight at RT. The reaction mixture was quenched in ice-cold water (1000 mL) and extracted with ethyl acetate (250 mL×3). The combined organic layers were washed with water (200 mL×3), brine solution (100 mL) and washed under Na 2 SO 4 Dry on top. The organic phase was concentrated under vacuum to obtain the crude compound, which was further purified by flash chromatography using (ethyl acetate:n-hexane (80:20)) to provide 1-[1-(1-methyl-1H-pyrazole -4-ylmethyl)-4-f...
Embodiment 2
[0344] N-[(1S,2S)-2-Hydroxycyclohexyl]-1-(2-chloropyridin-4-ylmethyl)-4-fluoro-1H-indole-3-carboxamide
[0345]
[0346] Step 1: 4-Fluoro-1H-indole-3-carboxylic acid
[0347]
[0348] To 2,2,2-trifluoro-1-(4-fluoro-1H-indol-3-yl)-ethanone (1-10, 18.49 g, 0.080 mol) was added 4N aqueous NaOH (200 mL, 0.80 mol), and heated to 100°C for 3 hours. The reaction mixture was cooled to RT and diluted with ice-cold water (200 mL). The aqueous layer was washed with ethyl acetate (100 mL×2) and acidified to pH about 4 with dilute HCl. The obtained solid was filtered and washed with 100 mL of water and n-hexane, respectively. These solids were dried under vacuum.
[0349] Yield: 5.43g (38%); 1 H-NMR (DMSO-d 6 , 400MHz) δppm: 6.84-6.89(m, 1H), 7.12-7.17(m, 1H), 7.26-7.28(m, 1H), 8.02(s, 1H), 11.87(s, 1H), 12.05(m, 1H);
[0350] Mass (m / z): 180.2 (M+H) + .
[0351] Step 2: N-[(1S,2S)-2-Hydroxycyclohexyl]-4-fluoro-1H-indole-3-carboxamide
[0352]
[0353] in N 2 To a solu...
Embodiment 3 to 19
[0360] Examples 3 to 19: The compounds of Examples 3 to 19 were prepared by following the experimental procedures described in Examples 1 and 2 with some minor changes.
[0361]
[0362]
[0363]
[0364]
[0365]
[0366]
[0367]
[0368]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com