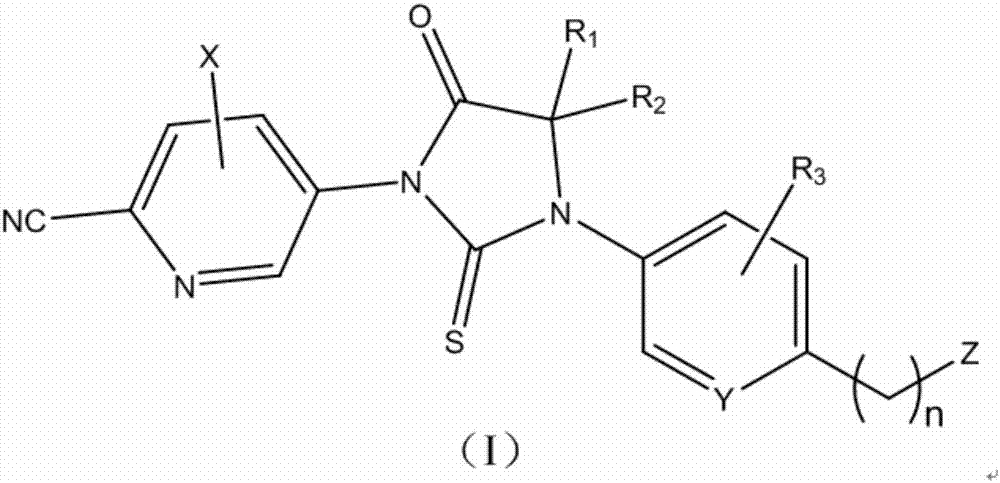
Sulfo-imidazole diketone androgen receptor antagonist and applications thereof
A technology of thioimidazolidine and halogen, which is applied in the field of medicine and can solve the problems of reduced affinity activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
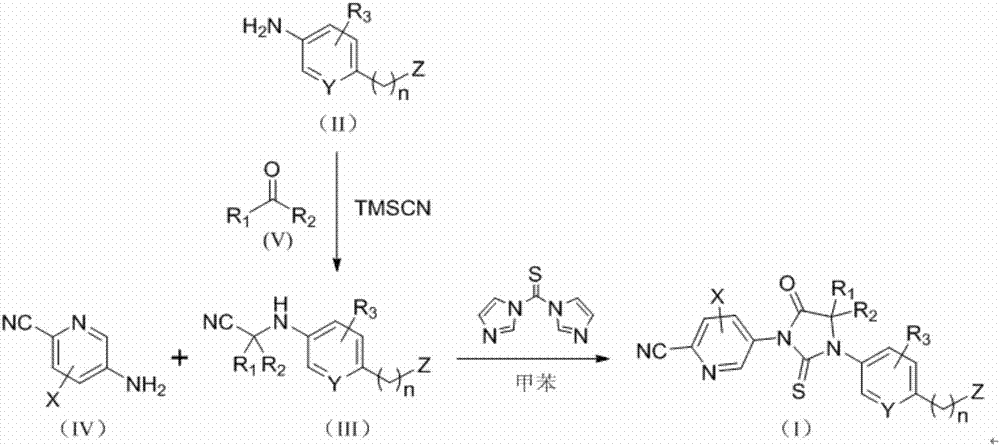
[0048] The preparation of compounds of formula (I) according to the invention is illustrated by Scheme A.
[0049] Option A:
[0050]
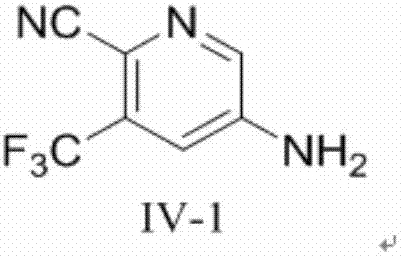
[0051] In scheme A, the compound of formula (IV) is non-limitingly selected from compound IV-1, which can be obtained commercially.
[0052]
[0053] In scheme A, the compound of formula (V) is non-limitingly selected from acetone, cyclobutanone and dihydro-3(2H)-furanone.
[0054] In certain embodiments, the compound of formula (II) is selected from: Y is carbon, R 3 Is amino meta-fluorine, Z is cyano, COOCH 3 、CONH 2 、CONHCH 3 , imidazol-2-yl or oxazol-2-yl.
[0055] In certain embodiments, the compound of formula (II) is selected from: Y is nitrogen, R 3 is hydrogen, Z is COOCH 3 、CONH 2 , oxazol-2-yl, thiazol-2-yl, or
[0056]
Embodiment 1
[0058] 4-(4-(3-(6-cyano-5-(trifluoromethyl)pyridin-3-yl)-5,5-dimethyl-4-oxo-2-thioimidazolidine-1 Preparation of -yl)-2-fluorophenyl)butanoic acid methyl ester (WX-101)
[0059]
[0060] Preparation of compound II-1: add compound 1 (12.05g, 0.05mol), ammonium chloride (45g), iron powder (30g), methanol (350mL) and water (250mL) into the reaction flask, stir, heat to reflux and Leave on for 1 hour. Cool down, filter, concentrate under reduced pressure to remove methanol, add dichloromethane for extraction. The organic phase was washed with brine, and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain compound II-1 (9.10 g, yield 86.1%) as light yellow oil. 1 H-NMR (400MHz, CDC1 3 ): δ1.83-1.88(m,2H), 2.32(t,2H,J=7.2Hz), 2.52(t,2H,J=7.2Hz), 3.63(s,3H), 5.44(bs,2H) , 6.31-6.37 (m, 1H), 6.90-6.98 (m, 2H).
[0061] Preparation of compound III-1: add compound II-1 (1.06g, 5mmol), zinc chloride (10...
Embodiment 2
[0064] 4-(4-(3-(6-cyano-5-(trifluoromethyl)pyridin-3-yl)-5,5-dimethyl-4-oxo-2-thioimidazolidine-1 Preparation of -yl)-2-fluorophenyl)butyramide (WX-102)
[0065]
[0066] Preparation of compound II-2: Add compound II-1 (2.11 g, 10 mmol) and methanol (30 mL) into a reaction flask, stir, add ammonia water (30 mL), and continue stirring for 24 hours. Concentrate under reduced pressure to remove methanol, add water to the residue, and extract with dichloromethane. The organic phase was washed with brine and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to obtain compound II-2 (1.54 g, yield 78.5%) as a white solid, which was directly used in the next step.
[0067] Preparation of compound III-2: According to the similar method for preparing compound III-1, compound III-2 (1.46 g) was synthesized from compound II-2 as light yellow oil, which was directly used in the next step.
[0068] Preparation of compound WX-102: According to the sim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com