Preparation method of octahydro dipyrroloquinoline compound
A technology for octahydrodipyrrole and compound, which is applied in the field of preparation of octahydrodipyrroloquinoline compounds, can solve the problems of pollution, high cost of chiral phosphoric acid and silver catalyst, and achieves the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 3-methoxyphenyl-2-pyrrolidinyl-1-ylphenylmethanol As an example, to prepare The reaction process is shown in Table 1,
[0023] 0.2mmol After being mixed with catalyst (20mol%), add in the solvent of 2ml, react under the condition of 60 ℃, reaction time is as shown in the table, check the productive rate of final product, the result is shown in table 1.
[0024] Table 1: Reaction conditions and results
[0025]
[0026] In 17 in Table 1, DCE is dried first, and the drying method is: add CaH to each liter of DCE 2 5g, distilled at 130°C; b : Column chromatography separation yield.
Embodiment 2
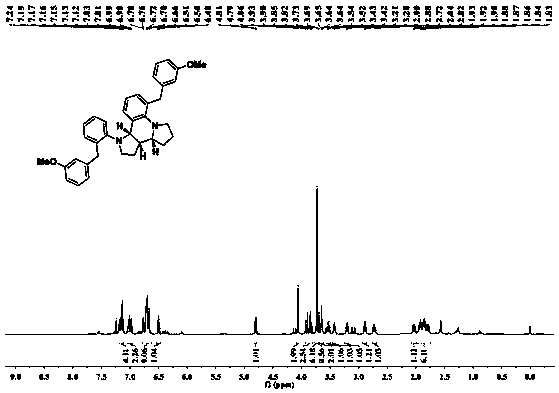
[0028] raw material: 3-methoxyphenyl-2-pyrrolidinyl-1-ylphenylcarbinol
[0029] Reaction conditions: Toluene, 60°C, PA (20%)
[0030] Product: Chemical formula: C 36 h 38 N 2 o 2
[0031] Exact molecular weight: 530.2933
[0032] Molecular weight: 530.7120
[0033] Structural formula:
[0034] Yield: 96%, dr 5:1.
[0035] 1 H NMR (500MHz, CDCl 3 )δ7.15(dt, J=14.9, 7.7Hz, 4H), 7.04–6.96(m, 2H), 6.79–6.62(m, 9H), 6.50(t, J=7.5Hz, 1H), 4.80(d ,J=8.4Hz,1H),4.06(s,2H),3.94–3.81(m,3H),3.74(d,J=12.0Hz,6H),3.65(dd,J=10.2,5.4Hz,2H) ,3.53(dd,J=16.1,7.9Hz,1H),3.42(td,J=8.5,4.0Hz,1H),3.20(td,J=8.7,2.7Hz,1H),2.89(dd,J=15.8 ,8.4Hz,1H),2.73(td,J=10.6,2.9Hz,1H),2.07–2.00(m,1H),1.98–1.74(m,6H); 13 C NMR (125MHz, CDCl 3 )δ159.65,159.56,150.33,145.68,143.65,143.38,137.12,131.00,130.68,129.17,129.09,127.94,127.78,127.16,127.01,123.26,122.30,121.81,121.24,118.45,114.95,114.49,111.09,111.02,63.78 ,59.70,55.12,55.07,53.67,52.26,42.71,39.90,37.45,29.07,25.84,24.50; HRMS(ESI):calcd ...
Embodiment 3
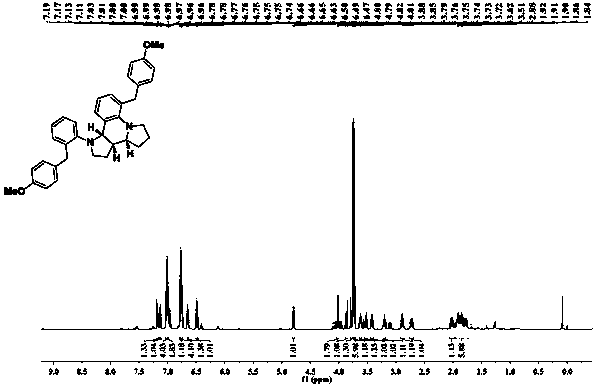
[0037] raw material: 4-Methoxyphenyl-2-pyrrolidin-1-ylphenylcarbinol
[0038] Reaction conditions: Toluene, 60°C, PA (20%)
[0039] Product: Chemical formula: C 36 h 38 N 2 o 2
[0040] Exact molecular weight: 530.2933
[0041] Molecular weight: 530.7120
[0042] Structural formula:
[0043] Yield: 92%, dr 5:1.
[0044] 1 H NMR (500MHz, CDCl 3 )δ7.20–7.15(m,1H),7.12(d,J=7.2Hz,1H),7.01(dd,J=9.0,5.2Hz,4H),6.99(dd,J=5.3,2.0Hz,2H ),6.80–6.78(m,1H),6.78–6.73(m,4H),6.65(dd,J=9.1,3.7Hz,1H),6.49(t,J=7.5Hz,1H),4.79(d, J=8.5Hz, 1H), 4.02(d, J=5.2Hz, 2H), 3.88(dd, J=10.6, 7.7Hz, 1H), 3.80–3.76(m, 1H), 3.74(d, J=6.0 Hz, 6H), 3.72(d, J=5.3Hz, 1H), 3.65–3.60(m, 1H), 3.52(dd, J=16.3, 7.8Hz, 1H), 3.42(td, J=8.6, 4.1Hz ,1H),3.23–3.18(m,1H),2.89(dt,J=8.7,6.9Hz,1H),2.72(qd,J=8.3,3.2Hz,1H),2.06–1.98(m,1H), 1.96–1.73(m,6H); 13 C NMR (125MHz, CDCl 3 )δ157.73,157.71,150.23,145.76,137.81,133.98,133.85,130.94,130.62,130.23,129.65,127.72,127.69,127.07,123.27,122.27,118.44,113.72,113...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com