Novel tlr4 antagonists
A sequence and receptor technology, applied in the field of novel TLR4 antagonists
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
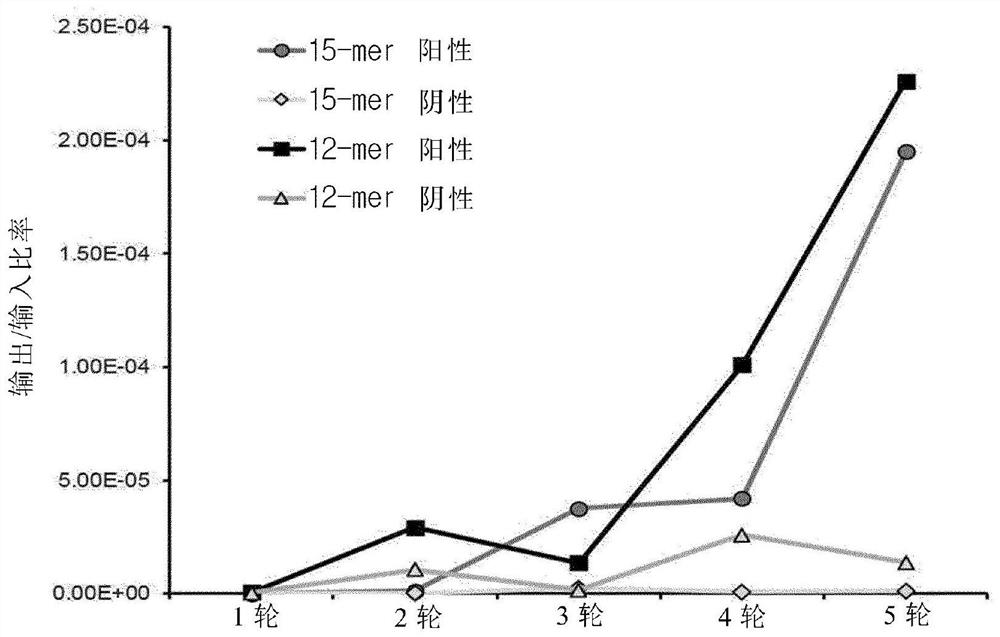
[0058] Example 1: Screening TLR4 / myeloid differentiation protein 2-specific peptides, in order to screen peptides that specifically bind to the TLR4 / myeloid differentiation protein 2 complex, fUSE55 was constructed as a 15-mer peptide, and pHEN2 was constructed as a 12-mer peptide The expression library was phage displayed and a phage display method was performed.
Embodiment 1-1
[0059] Example 1-1: Preparation of expression library
[0060] First, to prepare a 15-mer peptide expression library, use forward primer 5'-TTG ATC GCA AGG ATC GGCTAG C-3' reverse primer 5'-AA GGC CTT GGT ACC GCT GCC ACC(MNN)15 GCT AGC CGA TCC TTGCGA TCA A-3' and Pfu deoxyribonucleic acid (DNA) polymerase (SolGent, Daejeon, Korea) at 90°C, denatured for 30 seconds; at 55°C, annealed for 30 seconds; at 72°C Next, the process of extending for 60 seconds was repeated 25 times to amplify the deoxyribonucleic acid. After using NheI / KpnI to cut the amplified deoxyribose nucleic acid chain, use T4 deoxyribonucleic acid ligase (New England Biolabs, Inc., Ipswich, MA, USA) ) was ligated into the fUSE55 vector. Transfer 3 DNA expression libraries as electrocompetent E. coli cells (electrocompetent E.coli cells) DH10B strain, and finally prepare 6.6 × 10 7 After cloning, amplify and propagate in E. coli strain TG-1.
[0061] And, by preparing a random 12-mer peptide expression librar...
Embodiment 1-2
[0062] Embodiment 1-2: biological panning (Biopanning)
[0063] Biopanning was performed on a modified Griffin-1 library (Griffin H., MRC, Cambridge, UK, unpublished data). More specifically, Nunc Maxisorp 96-well plate (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA) was used in the coating buffer. MA, USA)) coated the resuspended recombinant human TLR4 / myeloid differentiation protein 2 complex (R&D Systems, Inc., Minneapolis, MN, USA) and refrigerated overnight. Then, under normal temperature conditions, after blocking with 1% bovine serum albumin (BSA) in phosphate buffered saline (PBS), under normal temperature conditions, the above-mentioned wells that were refrigerated were exposed to the phage expression library, thereby containing The concentration of bovine serum albumin in phosphate buffered saline (PBST) of Tween 20 (Tween 20) at a final concentration of 0.05% was 1%. The phage bound to the expression library was isolated with 100 μl of elution buffer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com