A kind of medicinal composition for treating heart disease
A pharmaceutical composition, a technology for heart disease, which can be used in drug combinations, pharmaceutical formulations, cardiovascular system diseases, etc., and can solve problems such as decreased cardiac function, inability to restore cardiac function, high blood sugar, and inability of damaged cells to regenerate by themselves.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: rat adipose stem cell extraction and experiment
[0052] Adipose stem cell extraction The fat in the abdominal cavity of 8-month-old Wistar strain male mice was removed by surgery, and the fat was cut into an appropriate size, and the fat was washed with physiological saline containing antibiotics, and the washed fat tissue was placed into Collagenase (0.01%) in physiological saline, heated and stirred in a 37°C water bath for about 1 hour, centrifuged at 3000rpm at room temperature for about 10 minutes, took out the lower sediment and placed it in a cell culture dish for cell culture.
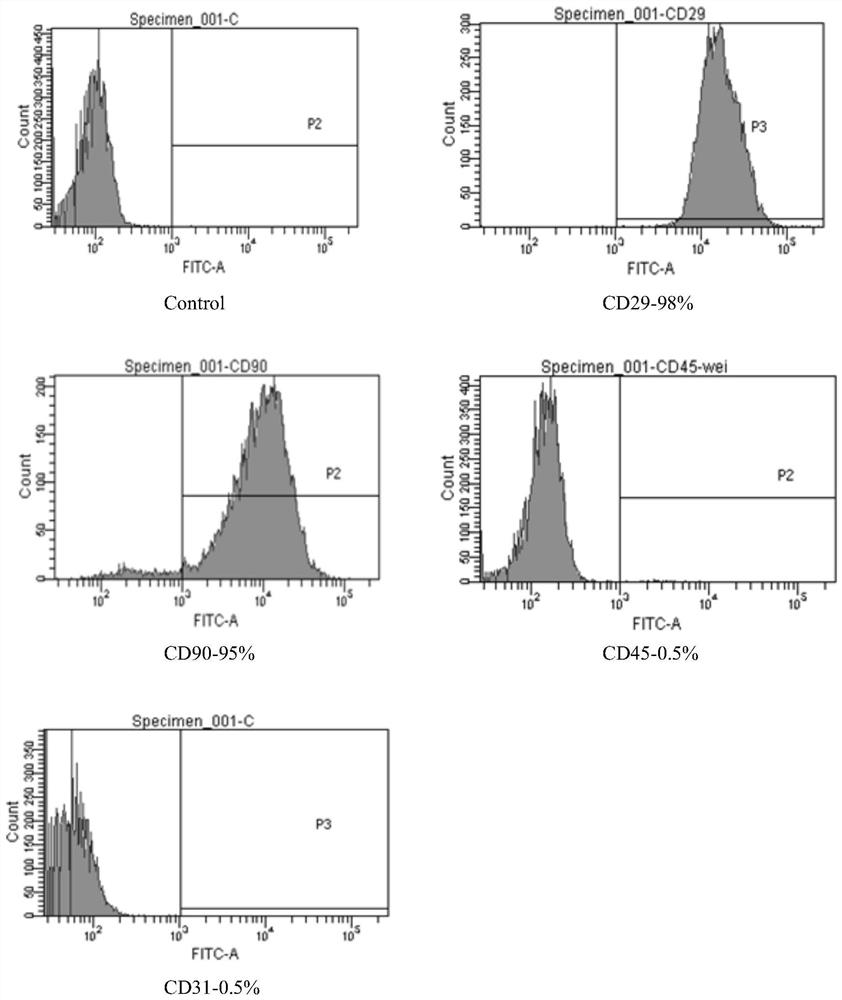
[0053] 1. Adipose stem cell identification experiment
[0054] Adipose stem cells are cultured to the second generation before being reinfused into mice through the tail vein for autologous adipose stem cell transplantation. Before stem cell autotransplantation, the cultured adipose stem cells need to be identified to confirm that the transplanted cells are stem cells. Th...
Embodiment 2
[0071] Embodiment 2: animal experiment design and analysis
[0072] Two-month-old Wistar strain male mice (purchased from Green Four Seasons Company) were divided into four groups, namely the normal group, the diabetic group induced by STZ (55 mg / kg), the diabetic plus autologous adipose stem cell treatment group, and the diabetic plus green tea EGCG pretreatment group. Autologous fat stem cell therapy group, etc. The rats were raised in the animal room under a cycle of 12 hours during the day and 12 hours at night. During the feeding period, they had free access to food and water. Two rats were kept in one animal cage, and the animal cage was changed every two days during the feeding period. When the blood sugar of the rats in the diabetic group rose to 200mg / dl, they were deemed to have diabetes, and the rats in the diabetic group were treated with autologous stem cell transplantation one month later. The reinfusion of autologous stem cells was 1×10 per rat through the ...
Embodiment 3
[0080] Embodiment 3: animal cardiomyocyte cell culture and analysis
[0081] Embryonic rat myocardial transformed cell line H9c2cells (from ATCC CRL-1446) and adipose stem cells were cultured in a medium containing 10% fetal bovine serum (fetal bovine serum, FBS, Hyclone), 1% Antibiotic-Antimycotic (antibacterial-antifungal agent, Gibco) Dulbeco's Modified Eagle Medium (DMEM, Sigma), the incubator is set to 5% CO2, 37 °C constant temperature environment, and the accompanying culture solution is replaced 2 to 3 times a week. After culturing cardiomyocytes overnight with serum-free medium (serum-free medium), the cardiomyocytes were treated with drugs at different time points or at different drug concentrations.
[0082] 1. Survivin analysis of animal cardiomyocytes
[0083] After the animal experiment, the hearts of the rats in each group were dissociated and homogenized, and the expression levels of proteins related to survival in the heart tissue of the rats in each group we...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap