Applications of pyrimidine compounds in preparation of medicines for promoting small intestine peristalsis
A technology of small intestinal peristalsis and pyrimidine, which is applied in the field of medicine and achieves the effect of good development and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: The compound 2-anilino-4-pyrrolidinyl-6-(5-(4-methyl-1-piperazinyl)-2-pyridyl)aminopyrido[3,4 -d] Preparation of pyrimidine tablets:
[0016] Prepare 2-phenylamino-4-pyrrolidinyl-6-(5-(4-methyl-1-piperazinyl)-2-pyridyl)aminopyrido[3,4 according to the method disclosed in Example 1 of CN107245075A -d] pyrimidine, take 20 grams of compound 2-anilino-4-pyrrolidinyl-6-(5-(4-methyl-1-piperazinyl)-2-pyridyl)aminopyrido[3,4 -d] pyrimidine, add 180 grams of conventional adjuvants for preparing tablets, mix evenly, and make 1000 tablets with a conventional tablet press.
Embodiment 2
[0017] Example 2: The compound 2-anilino-4-pyrrolidinyl-6-(5-(4-methyl-1-piperazinyl)-2-pyridyl)aminopyrido[3,4 -d] the preparation of pyrimidine capsules:
[0018] Prepare 2-phenylamino-4-pyrrolidinyl-6-(5-(4-methyl-1-piperazinyl)-2-pyridyl)aminopyrido[3,4 according to the method disclosed in Example 1 of CN107245075A -d] pyrimidine, take 20 grams of compound 2-anilino-4-pyrrolidinyl-6-(5-(4-methyl-1-piperazinyl)-2-pyridyl)aminopyrido[3,4 -d] pyrimidine, add conventional auxiliary materials for preparing capsules such as 180 grams of starch, mix well, and make 1000 capsules.
[0019] The following pharmacodynamic experiments will further illustrate its drug activity.
experiment example
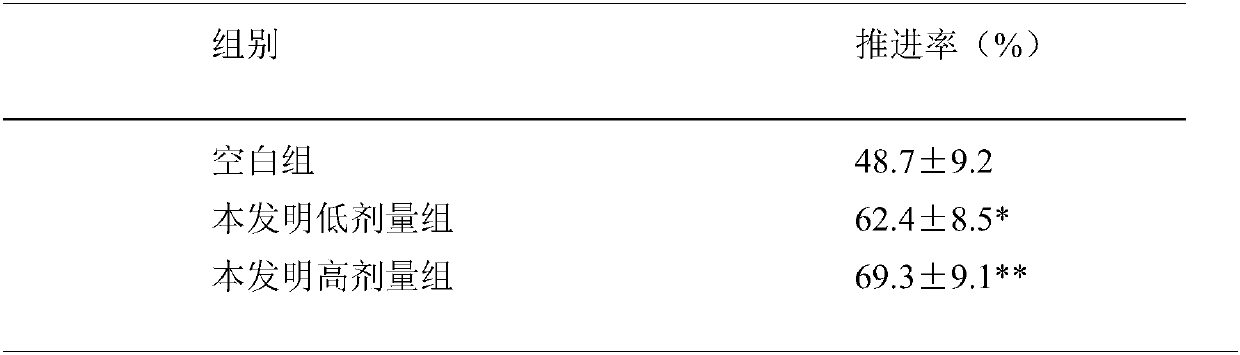
[0020] Experimental example: Evaluation of the compound 2-anilino-4-pyrrolidinyl-6-(5-(4-methyl-1-piperazinyl)-2-pyridyl)aminopyrido[3,4 -d] Effect of pyrimidine on propulsive motility of the small intestine
[0021] 1. Method: Get 30 Kunming mice, 20 ± 5g, half male and half male, and divide them into 3 groups randomly according to sex and body weight, i.e. blank group, 2-phenylamino-4-pyrrolidinyl-6-(5-( 4-Methyl-1-piperazinyl)-2-pyridyl)aminopyrido[3,4-d]pyrimidine (0.06mg / 10g, 0.24mg / 10g) two dose groups. 10 per group, 2-anilino-4-pyrrolidinyl-6-(5-(4-methyl-1-piperazinyl)-2-pyridyl)aminopyrido[3,4-d]pyrimidine Suspension was made with 5% charcoal powder and 10% gum arabic, and the blank group was directly suspended with charcoal powder and gum arabic without adding drugs, and both were prepared with normal saline. The mice were fasted for 24 hours before the experiment. Mice were intragastrically administered 0.2ml / 10g and killed 30 minutes after administration. Take ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com