Preparation method of anagrelide derivative
A technology for derivatives and substances, applied in the field of drug synthesis, can solve the problems of unreported synthetic methods of anagrelide derivatives, and achieve the effects of good environmental protection effect, reasonable process design, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
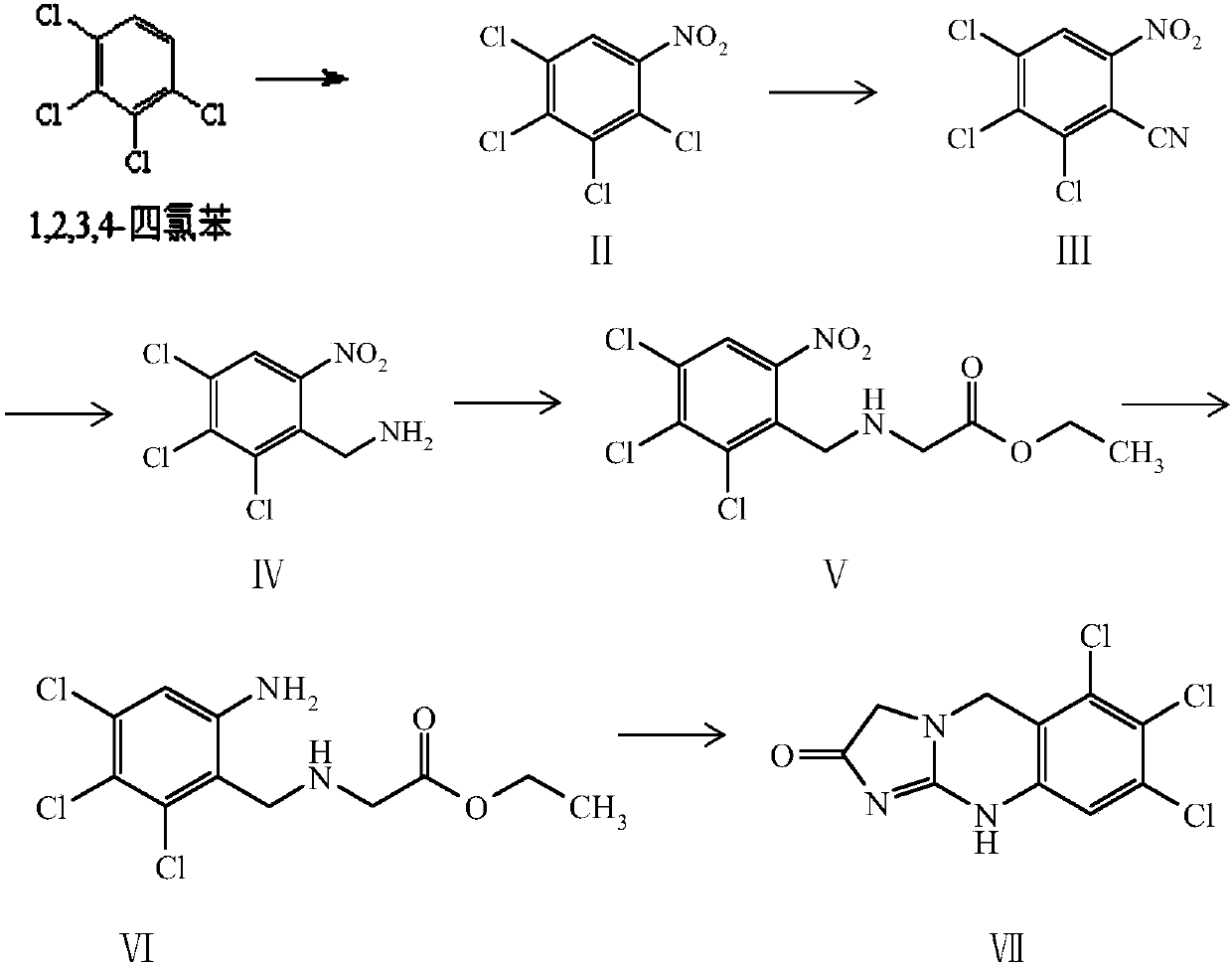
[0023] Preparation of Compound II: Suspend 1,2,3,4-tetrachlorobenzene in 40mL 98% concentrated sulfuric acid, add a mixed solution of 65% concentrated nitric acid and 9.17mL 98% concentrated sulfuric acid at a certain After stirring at high temperature for 3 hours, the reaction solution changed from a white suspension to a yellow suspension. Pour the reaction solution directly into 200g of ice, add 200mL of water, extract twice with 200mL of ethyl acetate, combine the organic phases, wash with 200mL of water, then wash with 200mL of saturated sodium bicarbonate, dry the organic phase, and evaporate to dryness The ratio of the amount of yellow solid compound II, 1,2,3,4-tetrachlorobenzene to concentrated nitric acid, and the experimental data of the reaction solvent are as follows:
[0024]
[0025] The screening experiment data of optimal reaction temperature are as follows:
[0026]
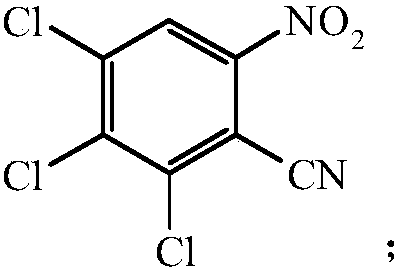
[0027] Preparation of Compound III: Dissolve 0.07 mol of Compound II in 9.6 mL of sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com