A class of isoindolinone compounds derived from marine fungi, their preparation methods and their application in the preparation of anti-inflammatory drugs
A technology of isoindolinone and marine fungi, applied in the field of pharmaceutical compounds, can solve problems such as adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for preparing isoindolinone compounds derived from marine fungi. The isoindolinone compounds are isolated from the fermentation broth of the marine fungus Diaporthe sp.SYSU-HQ3. The marine fungus Diaporthe sp.SYSU-HQ3 was isolated from the fresh leaves of Excoecaria agallocha L., a mangrove plant in the sea area of Zhuhai, Guangdong.
[0027] The marine fungus Diaporthe sp.SYSU-HQ3 was deposited in the Guangdong Provincial Microbial Culture Collection Center (GDMCC) on August 4, 2017, with a preservation number of GDMCC No: 60217 and a classification name of Diaporthe sp. The address of the preservation unit is 5th Floor, Building 59, Compound, No. 100 Xianlie Middle Road, Guangzhou City.
[0028] The specific preparation method of isoindolinone compound is as follows:
[0029] S1. Seed solution culture of marine fungus Diaporthe sp.SYSU-HQ3: insert marine fungus Diaporthesp.SYSU-HQ3 into seed medium, and cultivate in shaker at 180 rpm at 30°C for 6 days to ...
Embodiment 2
[0033] Structural testing and analysis of new compounds I, II, III, and IV yielded the following experimental data:
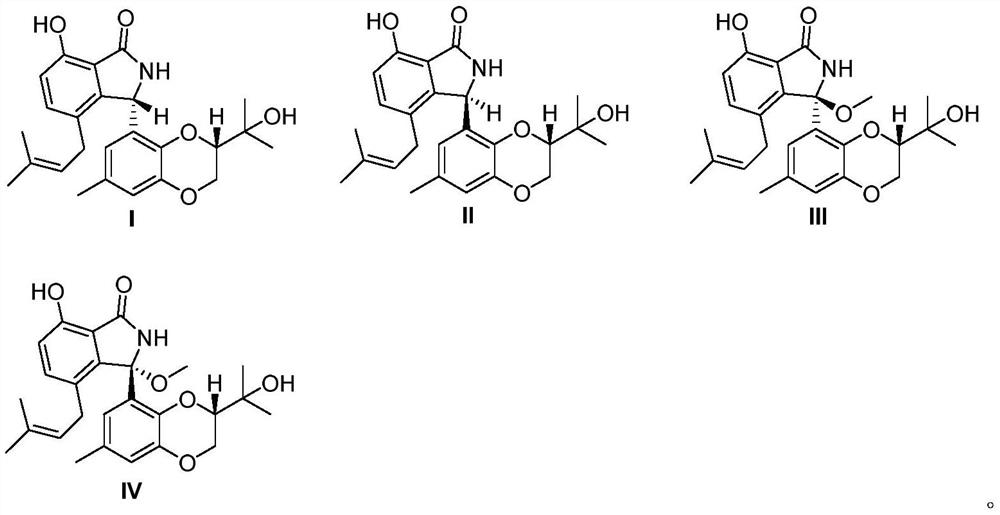
[0034] New compound I:C 25 h 29 NO 5 , HRESI-MS: 424.2123 [M+H] + (computed value 424.2124);
[0035] New compound II:C 25 h 29 NO 5 , HRESI-MS: 424.2124[M+H] + (calculated value 424.2126).
[0036] New compound III: C 26 h 32 NO 6 , HRESI-MS: 454.2234 [M+H] + (calculated value 454.2230).
[0037] New compound IV: C 26 h 32 NO 6 , HRESI-MS: 454.2233 [M+H] + (calculated value 454.2230).
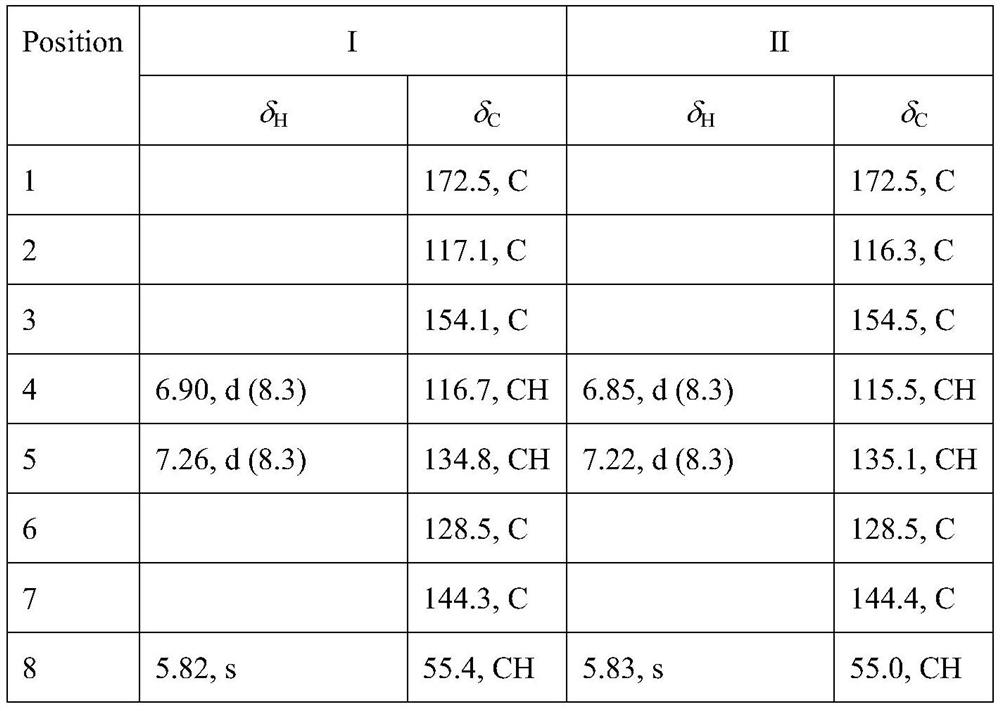
[0038] See Table 1 and Table 2 for the NMR data of compounds I, II, III, and IV.
[0039] Table 1. NMR data of compounds I and II (CDCl 3 , 500MHz / 125MHz,ppm)
[0040]
[0041]
[0042] Table 2. NMR data of compounds III and IV (CDCl 3 , 500MHz / 125MHz,ppm)
[0043]
[0044]
[0045] According to above-mentioned data result, confirm compound I, II, III, the structural formula of IV is as follows:
[0046]
Embodiment 3
[0048] Anti-inflammatory cell screening model of compound I-IV
[0049] 1. Cell culture and treatment: RAW 264.7 cells were cultured in vitro, using DMEM high-glucose medium containing 10% FBS, at 37°C and 5% carbon dioxide concentration for routine maintenance and passage.
[0050] 2. Compound intervention: adjust the RAW 264.7 cell density to 1×10 5 cells / well and in the logarithmic growth phase, adding LPS (final concentration 1 μg / mL) to induce macrophages to be in an inflammatory state, using DMSO to prepare the compound to be tested or indomethacin into different drug concentrations, each concentration was set at 3 A parallel well was set up, and positive control wells (only LPS added), negative control wells (cells and medium), and blank control wells (medium) were set. After culturing for 24 hours, take 50 μL of the cell supernatant and add it to a new 96-well plate, add 50 μL of reagents I and II of the NO detection kit, and use the Griess method to measure the conte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com