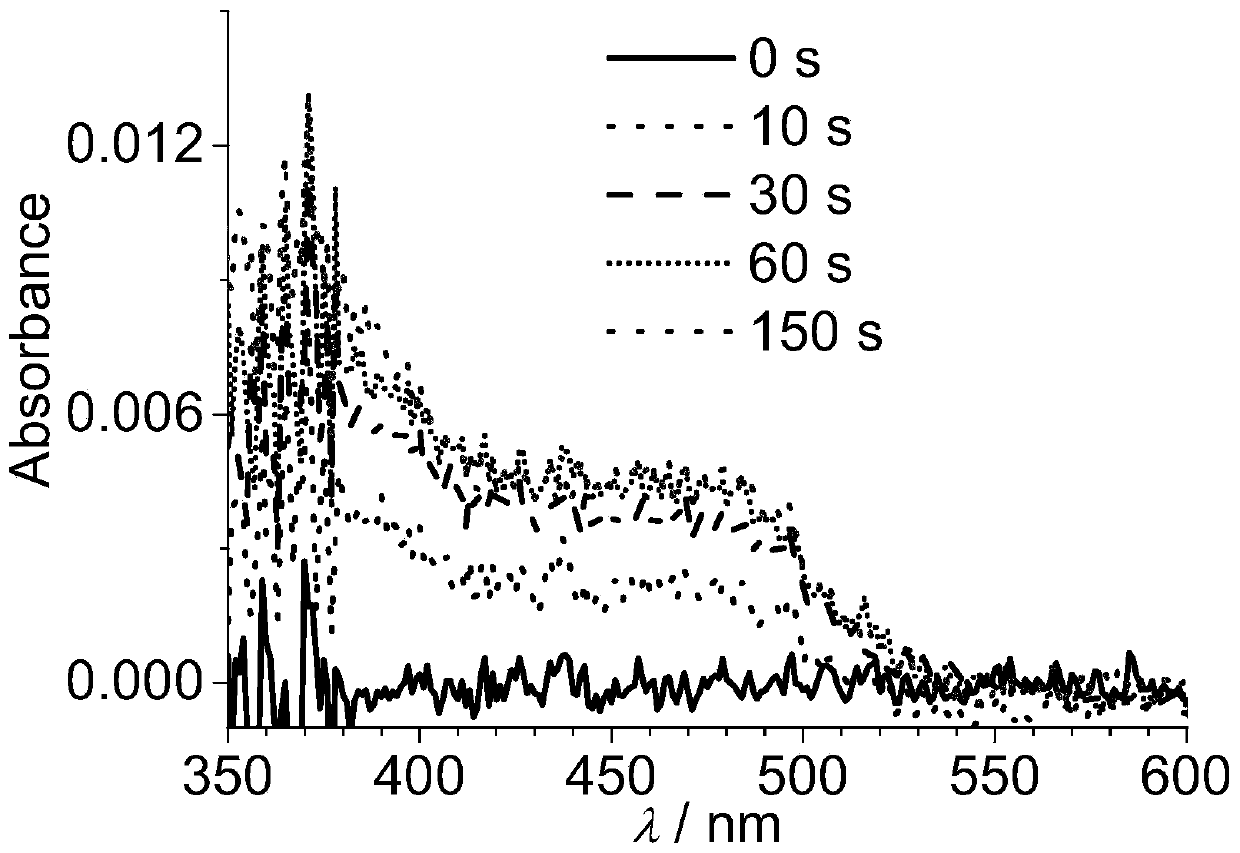
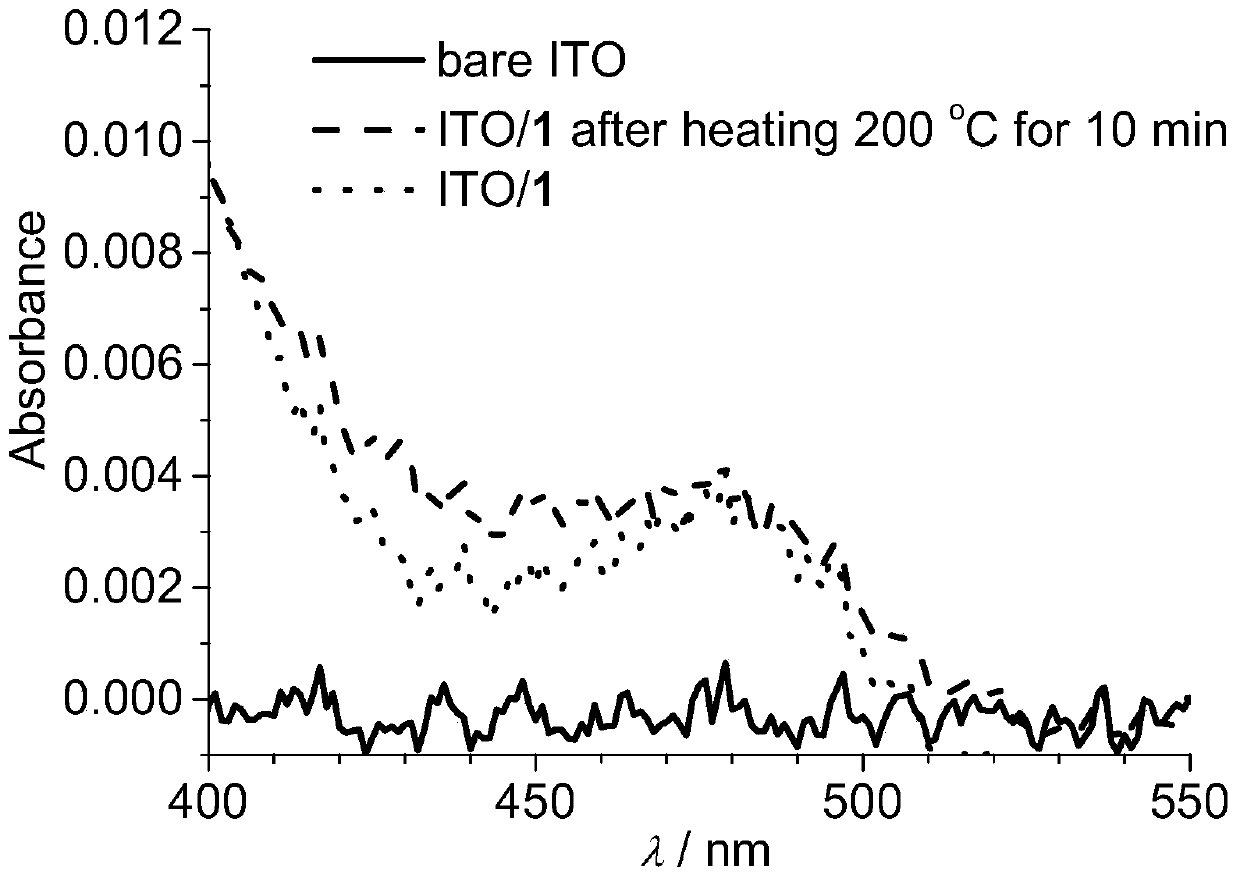
Tripod-shaped tetraaminepyrene and preparation method thereof, electrode modified by tripod-shaped tetraaminepyrene film and preparation method thereof
The technology of tripod-shaped tetraamine pyrene and tetraamine pyrene is applied in the field of electrode surface modification materials, which can solve the problems of poor stability, low surface adsorption efficiency, difficult synthesis of bridged molecular system, etc., and achieves high stability, low price of raw materials, Simple and efficient synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] Specifically, the preparation method of the above-mentioned tripod-shaped tetraamine pyrene comprises the following steps:
[0050] Step a: dissolving 1,3,6,8-tetrabromopyrene and diarylamine derivatives in an organic solvent to obtain a mixture of 1,3,6,8-tetrabromopyrene and diarylamine derivatives, wherein, 1 , the molar ratio of 3,6,8-tetrabromopyrene to diarylamine derivatives is 1:(4-10);
[0051] Step b: Add alkali and nitrogen to the mixture of 1,3,6,8-tetrabromopyrene and diarylamine derivatives to remove oxygen, and obtain 1,3,6,8-tetrabromopyrene and diarylamine after deoxygenation A mixture of arylamine derivatives, wherein the molar ratio of 1,3,6,8-tetrabromopyrene to base is 1:(4-10);
[0052] Step c: Add the palladium catalyst to the deoxygenated mixture of 1,3,6,8-tetrabromopyrene and diarylamine derivatives, and under nitrogen protection, 1,3,6,8-tetrabromopyrene and diarylamine The derivatives are refluxed at 90°C-140°C for 6h-48h, and the reaction ...
Embodiment 1
[0075] This embodiment provides a method for preparing tripod-like tetraaminepyrene having a structure shown in formula II, comprising the following steps:
[0076] Di(4-bromophenyl)amine (654mg, 2mmol), pyridine-4-boronic acid (1.23g, 10mmol), tetrakis(triphenylphosphine)palladium (231mg, 0.2mmol) and potassium carbonate (3.26g, 23.6 mmol) was dissolved in 20 mL of toluene, and heated to reflux for 24 hours under nitrogen protection. After the reaction, the system was cooled to room temperature, the solvent was removed under reduced pressure, and column chromatography separation and purification (eluent: petroleum ether / ethyl acetate 1 / 1, v / v) obtained diarylamine derivatives, producing The rate is 86%.
[0077] Dissolve 1,3,6,8-tetrabromopyrene (100mg, 0.20mmol) and diarylamine derivatives (484mg, 1.5mmol) in 10mL DMF to obtain 1,3,6,8-tetrabromopyrene and diarylamine Amine derivative mixture, wherein, the molar ratio of 1,3,6,8-tetrabromopyrene to diarylamine derivative i...
Embodiment 2
[0082] This embodiment provides a method for preparing tripod-like tetraaminepyrene having a structure shown in formula III, comprising the following steps:
[0083] Dissolve p-fluorobenzonitrile (1 g, 8.3 mmol), p-aminobenzonitrile (300 mg, 2.5 mmol), potassium tert-butoxide (28 mg, 0.25 mmol) in 20 mL DMSO, and stir for 24 hours under nitrogen protection. The reaction mixture was then poured into water and a precipitate formed. Filter, wash with water, and dry in vacuo to obtain a diarylamine derivative with a yield of 79%.
[0084] 1,3,6,8-Tetrabromopyrene (100mg, 0.2mmol) and diarylamine derivatives (263mg, 1.2mmol) were dissolved in 10mL DMF to obtain 1,3,6,8-tetrabromopyrene and diaryl Amine derivative mixture, wherein, the molar ratio of 1,3,6,8-tetrabromopyrene to diarylamine derivative is 1:6; Potassium phosphate (230mg, 1.1mmol) and nitrogen are added to 1,3,6 , 8-tetrabromopyrene and diarylamine derivative mixture are deoxygenated to obtain the deoxygenated 1,3,6,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com