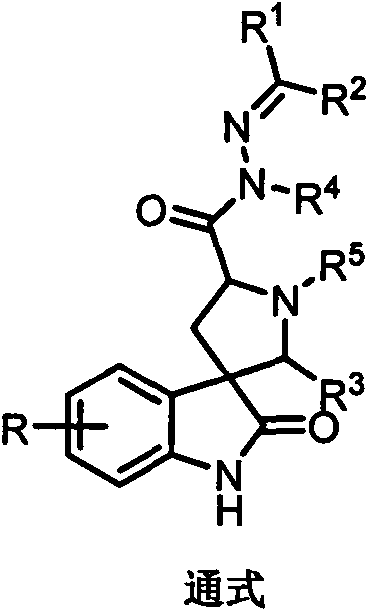
Spirooxoindole acylhydrazone derivative and preparation method thereof, and application of spirooxoindole acylhydrazone derivative in aspects of controlling plant viruses and killing bacteria and pests
A technology of indoleyl hydrazones and derivatives, applied in the field of pesticides, can solve the problems that the anti-plant virus activity, bactericidal activity and insecticidal activity are not reported in literature, etc., and achieve excellent anti-plant virus activity, high in vitro anti-TMV activity, The effect of good living activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
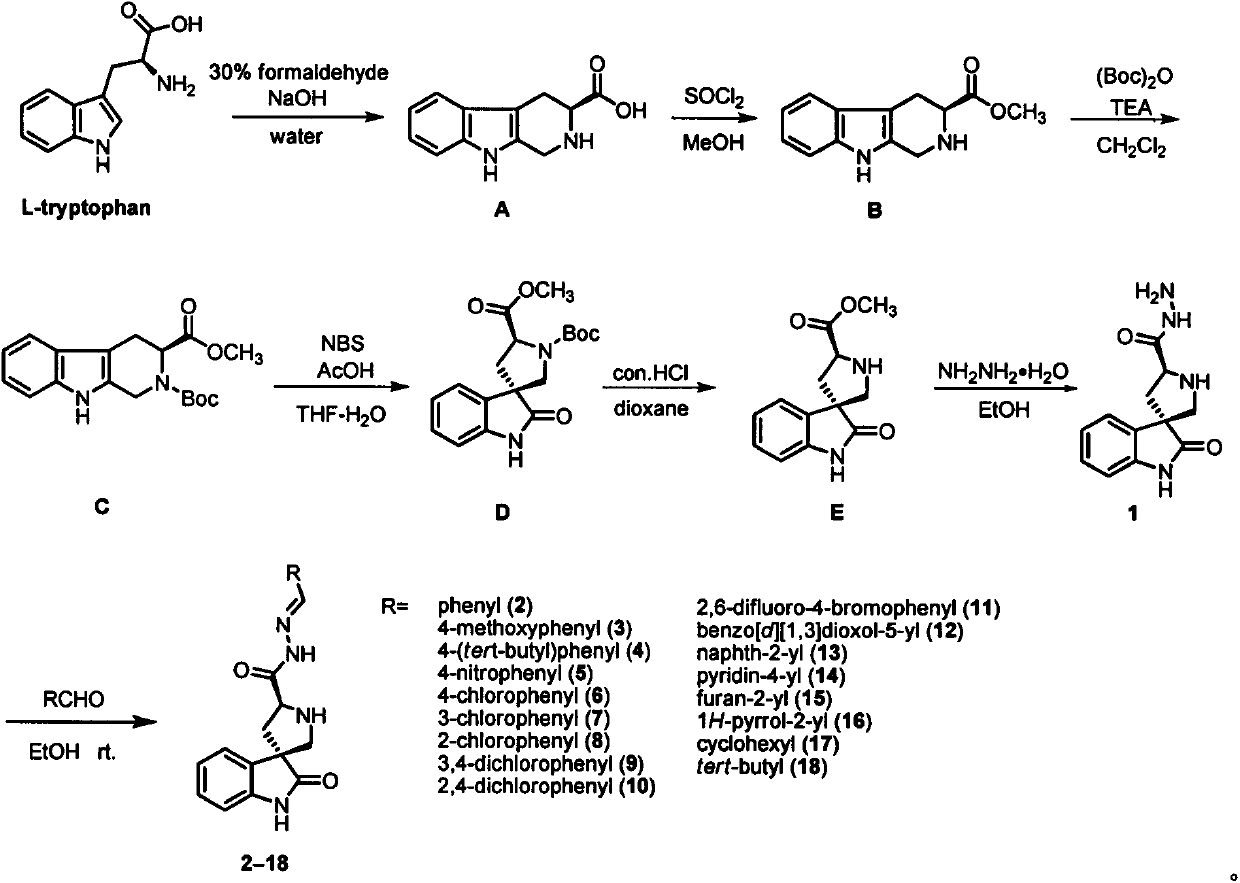
[0059] Embodiment 1: Synthesis (2-18) of hydrazide (1) and spirocyclic indole hydrazone derivatives
[0060]
[0061] Synthesis of (S)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid
[0062] Add L-tryptophan (30 g, 0.147 mol), NaOH (5.88 g, 0.147 mol) and 400 mL of water into a 1 L single-necked flask, and stir until clear. Then 18 mL of 30% formaldehyde aqueous solution was added, stirred at room temperature for 2 h, and then heated to reflux for 3 h. After cooling, the pH value was adjusted to 5 with 2 mol / L hydrochloric acid solution. At this time, a precipitate was formed, which was filtered, washed and dried to obtain 24.58 g of a khaki solid, with a yield of 77.4%. 1 H NMR (400MHz, DMSO-d 6 )δ10.96(s, 1H), 7.44(d, J=7.5Hz, 1H), 7.33(d, J=7.3Hz, 1H), 7.07(t, J=7.2Hz, 1H), 6.99(t, J=7.6Hz, 1H), 4.31-4.20(m, 2H), 3.64(dd, J=9.6, 4.4Hz, 1H), 3.35(brs, 1H), 3.15(dd, J=15.8, 4.4Hz, 1H ), 2.83 (dd, J=15.7, 10.7Hz, 1H).
[0063] Synthesis of (S)-2,3,4,9-tetr...
Embodiment 2
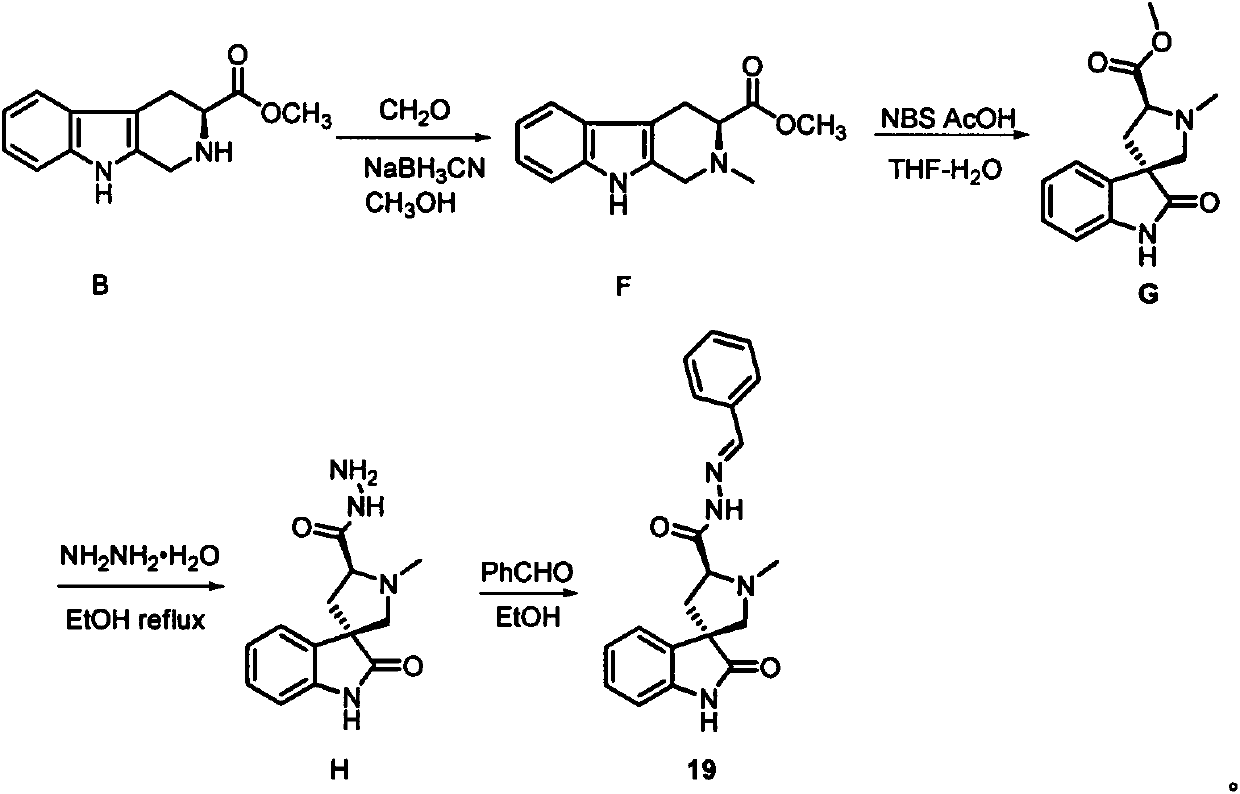
[0108] Embodiment 2: the synthesis (19) of spiro ring oxide indolehydrazone derivatives
[0109]
[0110] (3S,5'S)-N'-benzylidene-1'-methyl-2-oxospiro[indoline-3,3'-pyrrolidine]-5'-formylhydrazone (19)
[0111] Methyl β-carbolinecarboxylate B (4.10g, 17.8mmol) was dissolved in 50mL of methanol, and aqueous formaldehyde (5.0mL, 37wt% aq) sodium cyanoborohydride (2.79g, 44.5mmol) was added thereto under stirring conditions , stirred at room temperature for 2h. Then add saturated NaHCO to the reaction system 3 Aqueous solution (150 mL), the aqueous phase was extracted with ethyl acetate (3 x 75 mL), and the organic phases were combined. Wash the organic phase with saturated brine (30mL), anhydrous MgSO 4 Dry and desolvate under reduced pressure. Column chromatography separation (petroleum ether: ethyl acetate = 1:1) gave white solid F in 71% yield. 1 H NMR (400MHz, CDCl 3 )δ8.18(s, 1H), 7.46(d, J=7.3Hz, 1H), 7.23(d, J=8.4Hz, 1H), 7.15-7.05(m, 2H), 3.96(d, J=15.1 Hz, 1H)...
Embodiment 3
[0114] Embodiment 3: the synthesis (20-23) of spiro ring oxide indolehydrazone derivative
[0115]
[0116] (3S, 3'R, 7a'S)-2'-((E)-(4-chlorobenzylidene) amino)-3'-(4-chlorophenyl)-2', 3', 7 ',7a'-tetrahydrospiro[indoline-3,6'-pyrrolo[1,2-c]imidazole]-1',2(5'H)-dione (20)
[0117] Add 4-chlorobenzaldehyde (0.36 g, 2.56 mmol) and a catalytic amount of glacial acetic acid to a solution of hydrazide compound 1 (0.30 g, 1.22 mmol) in 35 mL of ethanol, heat the reaction solution to reflux for 3 h, and monitor the reaction by TLC. After the reaction was complete, the reaction liquid was concentrated under reduced pressure. Column chromatography (petroleum ether: ethyl acetate = 3:1) yielded compound 20 in 81%. White solid (dr=27:1), melting point 127-129°C. 1 H NMR (400MHz, CDCl 3 )δ9.84(s, 1H), 8.88(s, 1H), 7.61(d, J=8.3Hz, 2H), 7.48-7.27(m, 7H), 7.12-7.00(m, 2H), 6.84(d , J=7.5Hz, 1H), 5.76(s, 1H), 4.43(dd, J=9.2, 4.6Hz, 1H), 3.50(s, 2H), 2.82(dd, J=13.6, 4.6Hz, 1H) , 2.50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com