Use of a recombinant human cell line protein in the preparation of drugs for preventing or treating pulmonary fibrosis
A pulmonary fibrosis and cell line technology, which can be used in drug combinations, respiratory diseases, peptide/protein components, etc., can solve problems such as lack of rhCygb research reports, and achieve protection from oxidative stress damage, broad market prospects, good Social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 idiopathic pulmonary fibrosis modeling
[0021] 1. Experimental animals
[0022] Forty-five SPF grade adult male SD rats were purchased from Guangdong Medical Experimental Animal Center. The rats weighed 180-220 g, with an average of (198.35±19.28) g, and were randomly divided into two groups: 11 rats in the control group and 34 rats in the model group. Common feed was given under the condition of room temperature of 24°C and humidity of 78%.
[0023] 2. Main reagents and instruments
[0024] Bleomycin hydrochloride for injection (BLM, Nippon Kayaku Co., Ltd., 5mg / kg), batch number 430312
[0025] 2% pentobarbital solution (50mg / kg), batch number 69020100
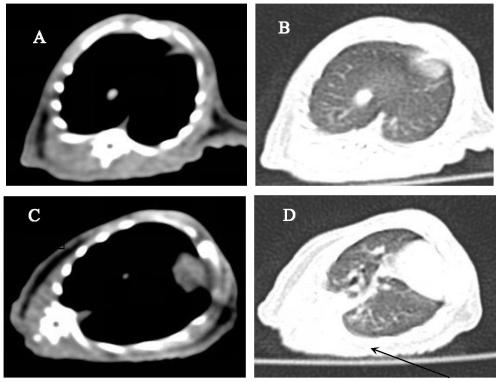
[0026] 16-layer spiral, model SomAToM Emotion CT, Siemens AG, Germany
[0027] Serum fiber four index detection reagents were purchased from Shenzhen New Industry Biomedical Engineering Co., Ltd., batch number 2013992021
[0028] Dexamethasone Sodium Phosphate Injection (Shenyang Yangguang Pharmace...
Embodiment 2
[0068] Example 2 Grouping and treatment of idiopathic pulmonary fibrosis
[0069] 1. Experimental grouping
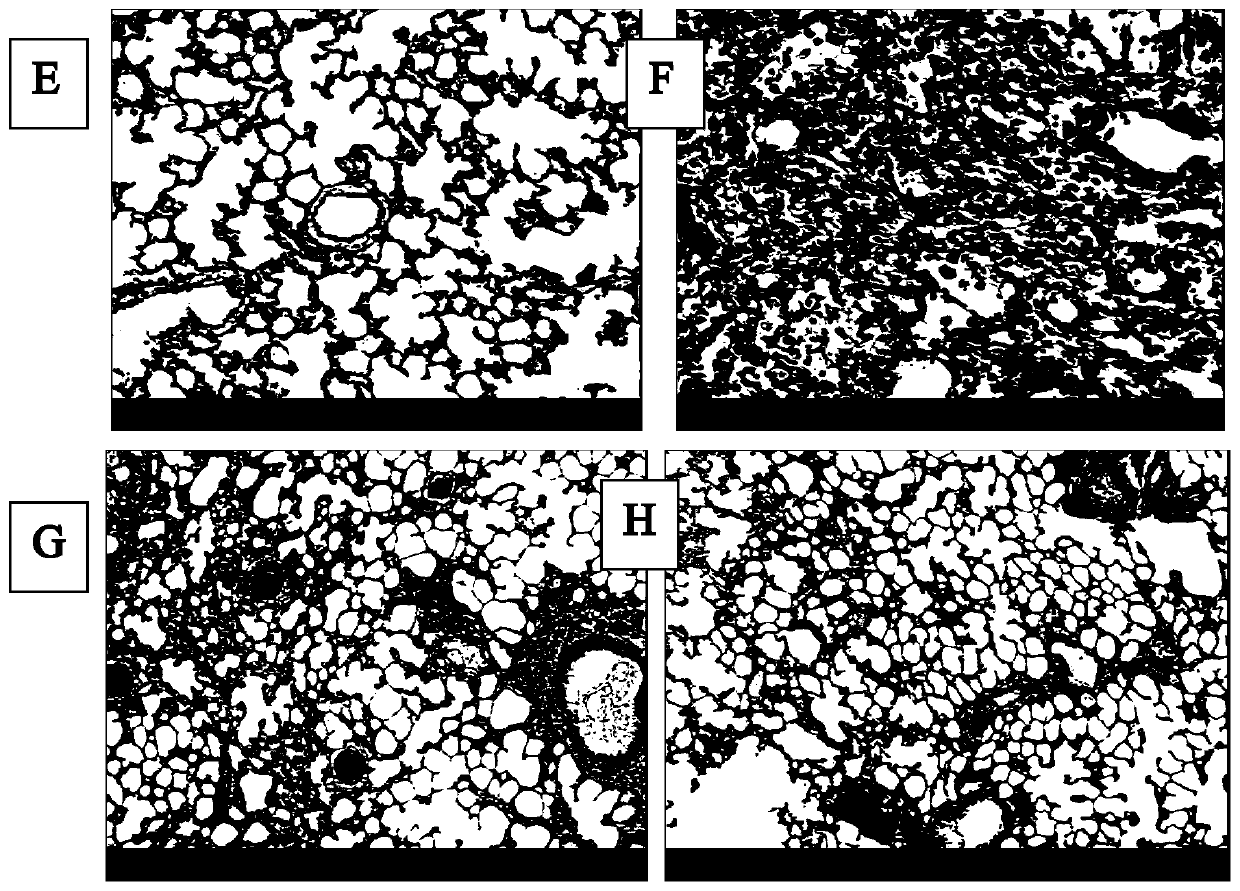
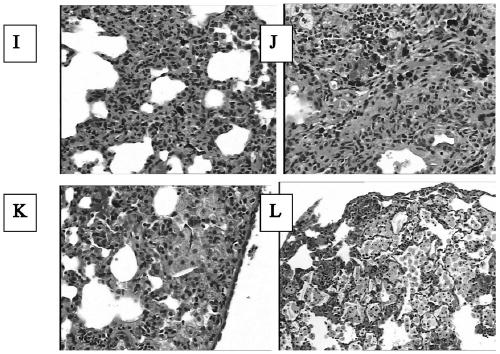
[0070] On the 2nd day after the successful modeling was confirmed by the first CT scan, that is, on the 8th day after the modeling, the model rats were randomly divided into a model group (group b, n=11), a dexamethasone treatment group (group c, n =11) and rhCygb group (group d, n=12). Group b was given normal feeding without any drug intervention; group c was given subcutaneous injection of dexamethasone injection 3 mg / kg daily from the 8th day after model establishment; group d was given subcutaneous injection of rhCygbd protein 4 mg daily from the 8th day after model establishment / kg (select CN 104096218 A patent to disclose recombinant human cell globin). On the 28th day and 56th day after the modeling, blood was collected from the tail vein of the rats to detect the four indicators of fibrosis. On the 56th day after the modeling, the second lung CT scan was p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com