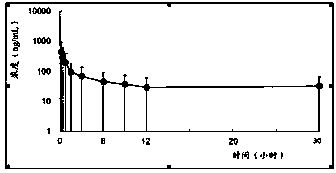
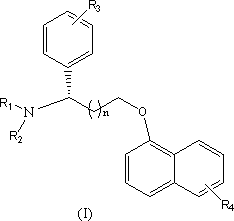
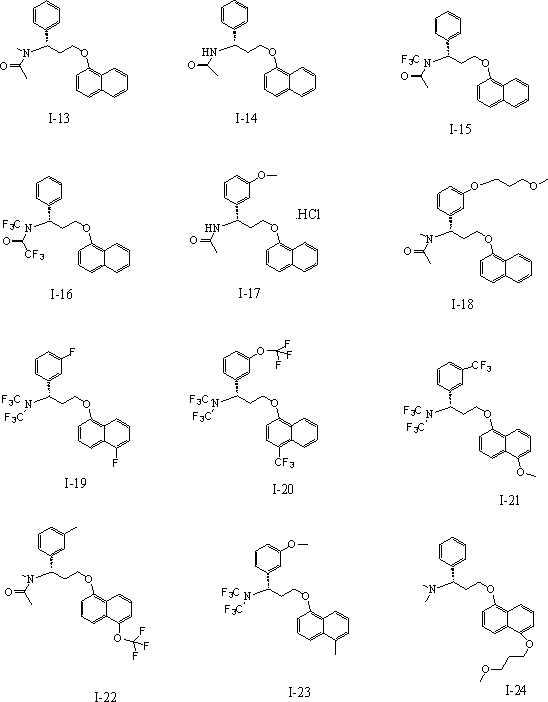
Novel 5-hydroxytryptamine reuptake inhibitor compounds as well as preparation method and applications thereof in medicine
A serotonin reuptake and inhibitor technology, applied in the field of medicinal chemistry, can solve problems such as increased severe arrhythmia, extrapyramidal side effects, increased serum prolactin levels, etc., and achieve improved oral utilization and extended half-life Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Preparation of (S)-1-(3-fluorophenyl)-N,N-dimethyl-3-(naphthalene-4-yloxy)propan-1-amine (compound I-1)
[0037]
[0038] Compound I-1
[0039] Take (S)-1-(3-fluorophenyl)-3-(naphthalene-4-yloxy)propan-1-amine 10g, add it into acetonitrile and stir to dissolve. Then 0.48 g of dimethyl sulfate was added. Heat to reflux for 6 hours. Add 1% activated carbon in total volume to the reaction liquid, reflux for decolorization for 15 min, and filter. The filtrate was concentrated to dryness under reduced pressure, and the residue was separated by HPLC to obtain 3.3 g of compound I-1.
Embodiment 2
[0040] Example 2: (S)-N,N-Dimethyl-3-(naphthalen-4-yloxy)-1-(3-(trifluoromethyl)phenyl)propan-1-amine (Compound I -2) Preparation
[0041]
[0042] Compound I-2
[0043] Take 10.0 g of (S)-3-(naphthalene-4-yloxy)-1-(3-(trifluoromethyl)phenyl)propan-1-amine, add it into acetonitrile and stir to dissolve. Then 0.48 g of dimethyl sulfate was added. Heat to reflux for 6 hours. Add 1% activated carbon in total volume to the reaction liquid, reflux for decolorization for 15 min, and filter. The filtrate was concentrated to dryness under reduced pressure, and the residue was separated by HPLC to obtain compound I-24.5g.
Embodiment 3
[0044] Embodiment 3: Preparation of (S)-3-(1-fluoronaphthalen-5-yloxy)-N,N-dimethyl-1-phenylpropane-1-amine (compound I-7)
[0045]
[0046] Compound I-7
[0047] Prepared according to the method of Example 1, except that (S)-1-(3-fluorophenyl)-3-(naphthalene-4-yloxy)propan-1-amine is replaced by (S)-3- (1-fluoronaphthalen-5-yloxy)-1-phenylpropan-1-amine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com