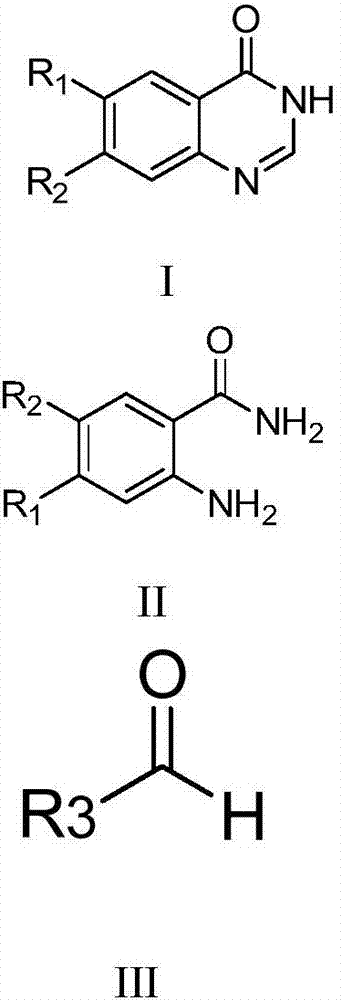
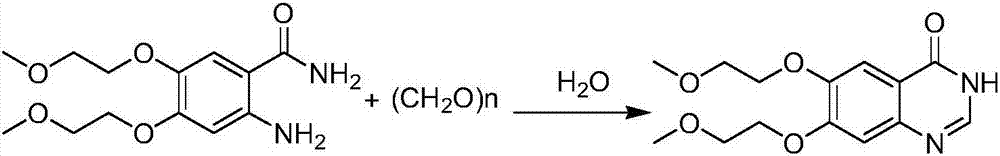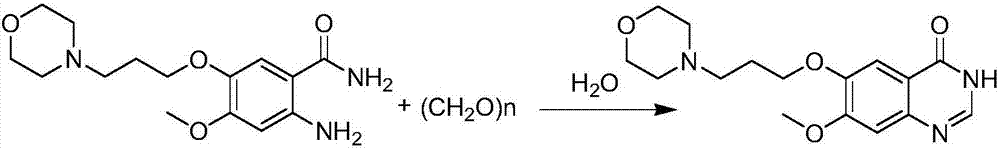Method for preparing compound
A compound and drug technology, applied in the field of chemistry, can solve problems such as intractable, toxic, and need for further development and research, and achieve the effect of equipment and environment friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 2-Amino-4,5-bis[(2-methoxy)-ethoxy]-benzamide (cas:236750-62-2, 2.84g, 10mmol) and paraformaldehyde (600mg, 20mmol mono Body) [monomer molar ratio: 1:2] was added to the reaction flask, then 10ml of water was added, sealed and heated to 130 degrees, reacted for 24 hours, then stood still, filtered out the solid, and recrystallized it with ethyl acetate , 2.70 g of 6,7-bis[(2-methoxy)-ethoxy]-quinazolinone can be obtained with a yield of 92%. The synthetic reaction formula is as follows:
[0024]
Embodiment 2
[0026] 2-Amino-4-methoxy-5-[(3-morpholinyl)-propoxy-]-benzamide (cas: 246512-44-7, 3.09 g, 10 mmol) was mixed with paraformaldehyde ( 600mg, 20mmol monomer) [monomer molar ratio: 1:2] was added to the reaction flask, then 10ml of water was added, sealed and heated to 130 degrees, reacted for 48 hours, then stood still, filtered out the solid, and washed it with acetic acid Ethyl ester was recrystallized to obtain 2.80 g of 7-methoxy-6-[(3-morpholinyl)-propoxy-]-quinazolinone with a yield of 88%. The synthetic reaction formula is as follows:
[0027]
Embodiment 3
[0029] 2-Amino-4,5-bis[(2-methoxy)-ethoxy]-benzamide (cas:236750-62-2, 2.84g, 10mmol) and paraformaldehyde (7.50g, 250mmol monomer) [monomer molar ratio: 1:25] was added to the reaction flask, then 10ml of water was added, sealed and heated to 130 degrees, reacted for 12 hours, then stood still, filtered out the solid, and weighed it with ethyl acetate Crystallize to obtain 2.64 g of 6,7-bis[(2-methoxy)-ethoxy]-quinazolinone with a yield of 90%. The synthetic reaction formula is as follows:
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com