Devices and methods for molecular diagnostic testing
A technology of reagents and amplicons, applied in the direction of sterilization methods, chemical instruments and methods, biochemical equipment and methods, etc., can solve the problems of late diagnosis of viral infection, loss of time, infection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
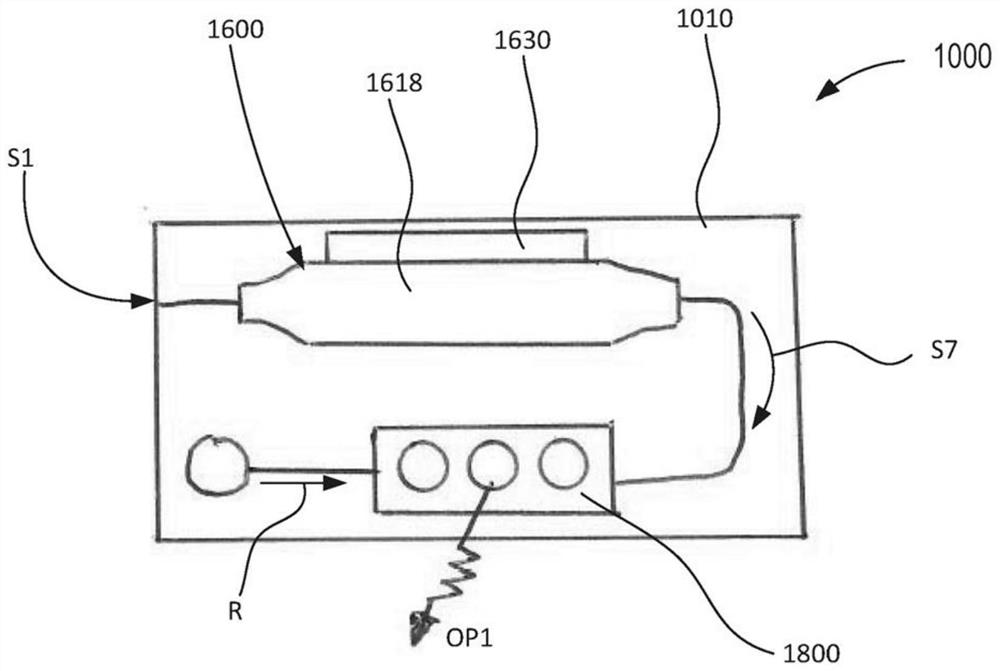
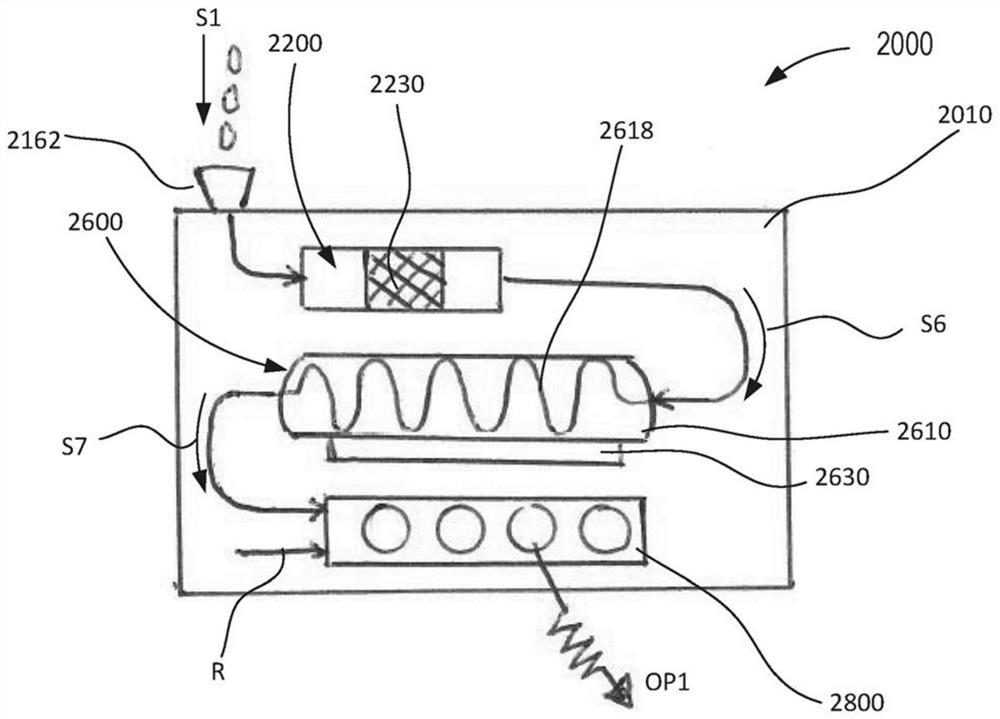
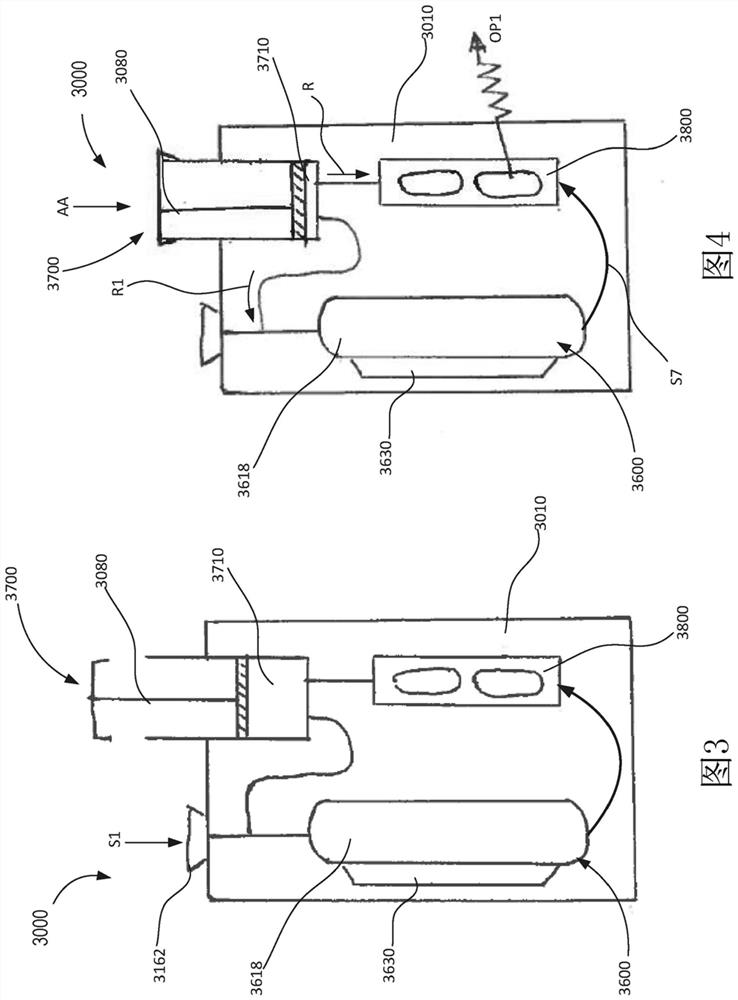
[0084] In some embodiments, the device is configured for a single-use, portable, single-use, inexpensive molecular diagnostic approach. The apparatus may include one or more modules configured to perform high quality molecular diagnostic tests including, but not limited to, sample preparation, nucleic acid amplification (eg, via polymerase chain reaction or PCR), and detection. In some embodiments, sample preparation can be performed by isolating target pathogens / entities and removing unwanted PCR inhibitors. The target entity can then be solubilized to release the target nucleic acid for PCR amplification. The target nucleic acid in the target entity can be amplified with a temperature cycled polymerase to generate higher copy numbers of the target nucleic acid sequence for detection.
[0085] In some embodiments, detection can occur by a colorimetric reaction in the read lane. Multiple nucleic acid targets can be read in a lane, allowing multiplexed detection / testing. The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com