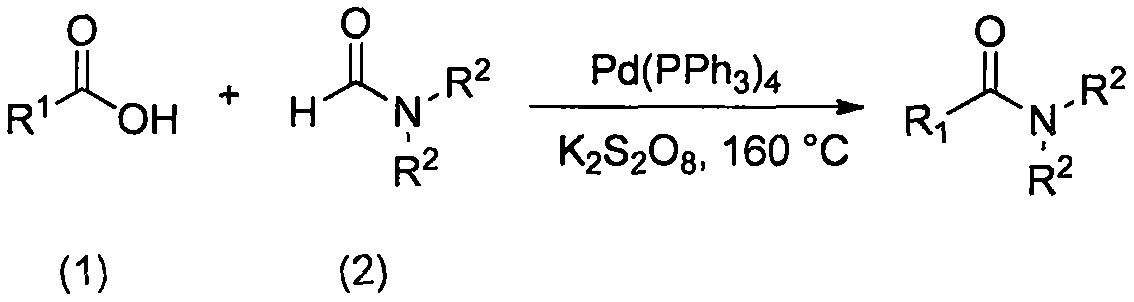
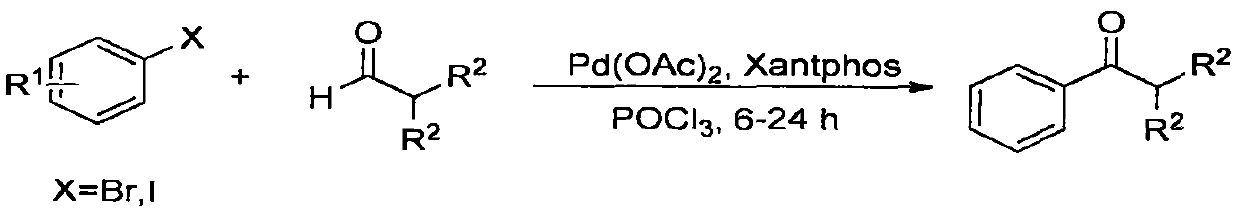A pd (pph 3 ) 4 Catalytic synthesis of amides
A technology of amide compounds and synthesis methods, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid amides, etc., can solve the problems of high toxicity and bad smell of aromatic hydrocarbons, and achieve simple operation and strong atom economy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of N,N-dimethylbenzamide from benzoic acid and DMF:
[0023]
[0024] Add benzoic acid (0.036g, 0.3mmol), Pd(PPh 3 ) 4 (0.017g, 0.015mmol), K 2 S 2 o 8 (0.162g, 0.6mmol), DMF (1.5mL), tighten the bottle cap, and react at an external temperature of 160°C for 18 h; monitor by gas chromatography; =4:1) After separation, a colorless transparent liquid was obtained with a yield of 75%.
[0025] 1 H NMR (300MHz, CDCl 3 )δ7.39(s, 5H), 3.10(s, 3H), 2.96(s, 3H); 13 C NMR (75MHz, CDCl 3 )δ171.76(s), 136.34(s), 129.61(s), 128.43(s), 127.11(s), 39.69(s), 35.43(s); MS(70eV, EI) m / z(EI) C 9 h 11 NO[M]: 149.19, 51(36), 77(100), 105(29), 148(56), 149(5).
Embodiment 2
[0027] Synthesis of N,N-dimethyl-2-chlorobenzamide from 2-chlorobenzoic acid and DMF:
[0028]
[0029] Add 2-chlorobenzoic acid (0.047g, 0.3mmol), Pd(PPh 3 ) 4 (0.017g, 0.015mmol), K 2 S 2 o 8 (0.162g, 0.6mmol), DMF (1.5mL), tighten the bottle cap, and react at 160°C for 18h at an external temperature; monitor by gas chromatography; 4:1) was separated to give a white solid in 80% yield.
[0030] 1 H NMR (300MHz, CDCl 3 )δ7.35-7.19 (m, 4H), 3.07 (s, 3H), 2.80 (s, 3H); 13 C NMR (75MHz, CDCl 3 )δ168.57(s), 136.43(s), 130.28(d, J=14.4Hz), 129.67(s), 127.85(s), 127.29(s), 38.18(s), 34.76(s); MS( 70eV, EI)m / z(EI)C 9 h 10 ClNO[M]: 183.63, 75(100), 111(84), 139(59), 182(16), 184(6).
Embodiment 3
[0032] Synthesis of N,N-dimethyl-2-trifluoromethylbenzamide from 2-trifluoromethylbenzoic acid and DMF:
[0033]
[0034] Add 2-trifluoromethylbenzoic acid (0.057g, 0.3mmol), Pd(PPh 3 ) 4 (0.017g, 0.015mmol), K 2 S 2 o 8 (0.162g, 0.6mmol), DMF (1.5mL), tighten the bottle cap, and react at 160°C for 18h at an external temperature; monitor by gas chromatography; 4:1) A white solid was obtained after isolation in 72% yield.
[0035] 1 H NMR (300MHz, CDCl 3 )δ7.70(d, J=7.8Hz, 1H), 7.62(dd, J=5.9, 3.4Hz, 1H), 7.51(t, J=7.7Hz, 1H), 7.35(d, J=7.5Hz, 1H), 3.14(s, 3H), 2.80(s, 3H); 13 C NMR (75MHz, CDCl 3 )δ169.09(s), 135.38(d, J=2.2Hz), 132.33(d, J=0.8Hz), 129.14(s), 127.42(s), 126.70(d, J=4.6Hz), 38.95( s), 34.93(s); MS(70eV, EI) m / z(EI)C 10 h 10 f 3 NO[M]: 217.19, 75(33), 95(37), 145(63), 173(61), 216(100), 217(14).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com