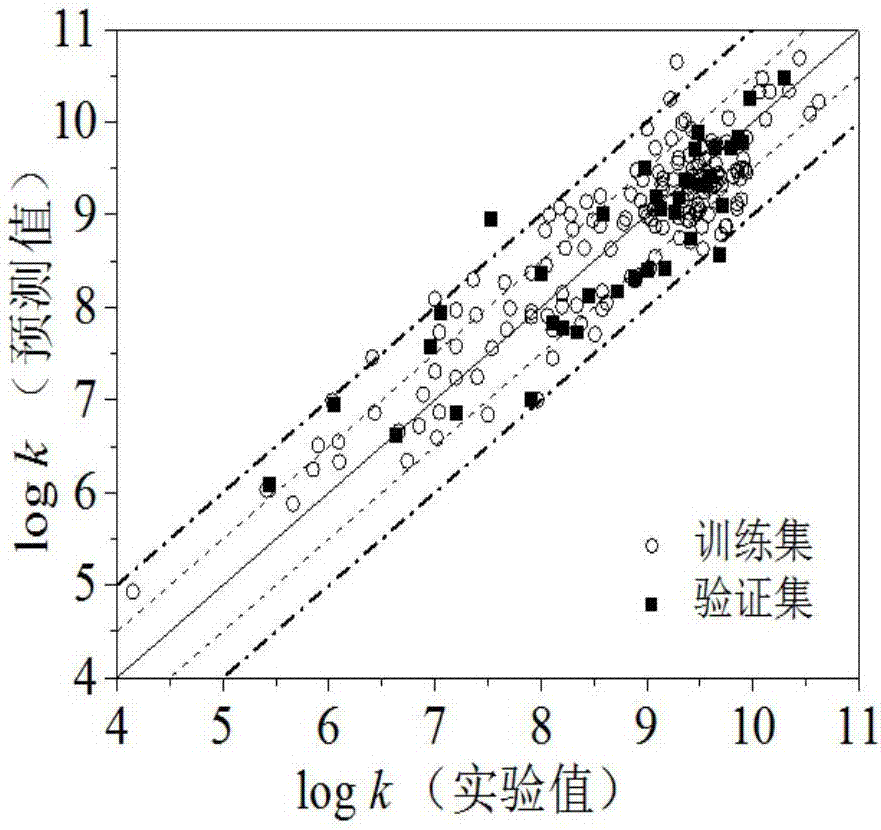
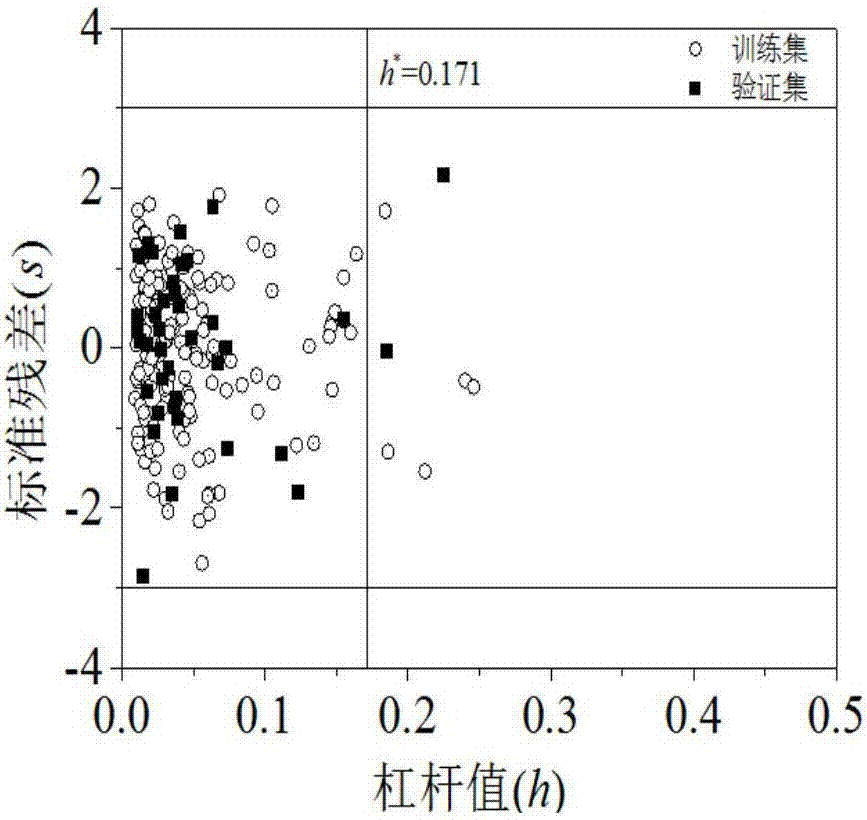
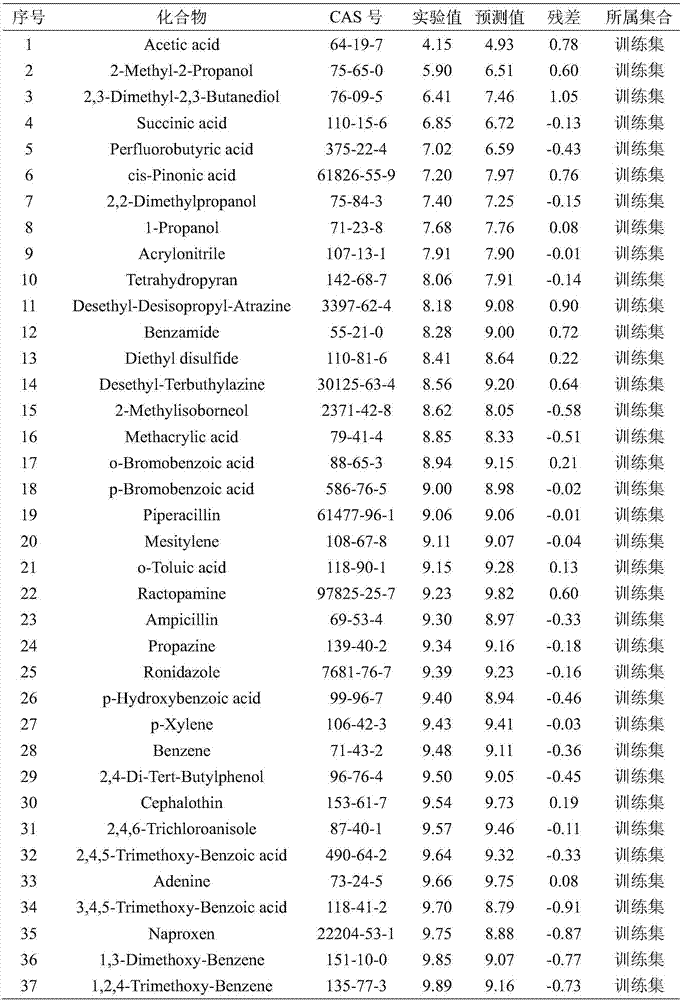
Quantitative structure activity relationship model for predicting water-phase reaction rate constant of organic matter and sulfuric acid free radical in water phase
A technology of reaction rate constant and quantitative structure, applied in measurement devices, computer materials science, electrical digital data processing, etc., can solve problems such as lack of calculation formulas, lack of models, difficulty in large batches, etc. The effect of low material consumption and good prediction ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Randomly given carboxylic acid compound succinic acid (CAS No. 110-15-6), predict its value. First optimize the molecular structure of succinic acid, and then based on the optimized molecular structure, calculate 8 kinds of molecular descriptors AVS_B(p), E HOMO , GATS3m, SaaaC, nArNO 2 , the values of MPC05, IC1 and SpMax_EA(dm) are 2.79, -11.506, 1.293, 0, 0, 1.609, 2.522, 0.334, respectively. h=0.036* , so the compound is in the application domain. Substituting the descriptor value into the built model, the calculation result is 6.72, the experimental value is 6.85, and the prediction result is good.
Embodiment 2
[0032] Randomly given the compound acrylonitrile (CAS No. 107-13-1) containing carbon-carbon double bond and cyano group, predict its value.
[0033] First optimize the molecular structure of acrylonitrile, and then based on the optimized molecular structure, calculate 8 kinds of molecular descriptors AVS_B(p), E HOMO , GATS3m, SaaaC, nArNO 2 , the values of MPC05, IC1 and SpMax_EA(dm) are 3.081, -10.982, 0.903, 0, 0, 0, 2.128, 0.678, respectively. h=0.131* , so the compound is within the application domain of the model. Substituting the descriptor value into the built model, the calculation result is 7.90, the experimental value is 7.91, and the prediction result is good.
Embodiment 3
[0035] The antibiotic compound sulfathiazole (CAS No. 72-14-0) containing amino and sulfur atoms is randomly given, and its value.
[0036] First optimize the molecular structure of sulfathiazole, and then based on the optimized molecular structure, calculate 8 kinds of molecular descriptors AVS_B(p), E HOMO , GATS3m, SaaaC, nArNO 2 , the values of MPC05, IC1 and SpMax_EA(dm) are 3.53, -8.886, 1.084, 0, 0, 3.555, 3.433, 0, respectively. h=0.032* , so the compound is within the application domain of the model. Substituting the descriptor value into the built model, the calculation result is 10.69, the experimental value is 10.44, and the prediction result is good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com