Application of nicotinamide adenine dinucleotide to prepare drug for treating inflammatory pain
A technology of nicotinamide adenine and dinucleotide, which is applied in the direction of drug combination, non-central analgesics, antipyretics, etc., can solve the side effects that limit clinical application, and the ineffectiveness of non-steroidal anti-inflammatory drugs on inflammatory pain Obvious issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Nicotinamide adenine dinucleotide has an analgesic effect on formalin-induced inflammatory pain. The following pharmacodynamic experiments further illustrate the role of nicotinamide adenine dinucleotide in formalin-induced inflammatory pain Has a therapeutic effect.
[0021] The experimental animals were 20g ICR mice, which were purchased from the Experimental Animal Center of Tongji University. Feeding conditions: standard feed, room temperature maintained at (24±2)°C, humidity 50-60%, daily light and dark time 12h each, before the experiment, The animals were placed in the experimental environment for 3 days to acclimatize.
[0022] Take 30 ICR mice (male, about 20g), and randomly divide them into five groups, 6 in each group, respectively: normal saline group (intrathecal injection, 10ul), morphine group (intrathecal injection, 350nmol / L, 10ul) , nicotinamide adenine dinucleotide group (Sigma-Aldrich company, the concentration is 8mmol / L, 80mm / L, 800mmol / L respecti...
Embodiment 2
[0024] Nicotinamide adenine dinucleotide has an analgesic effect on inflammatory pain in mice induced by complete Freund's adjuvant, which will be further explained through pharmacodynamic experiments below.
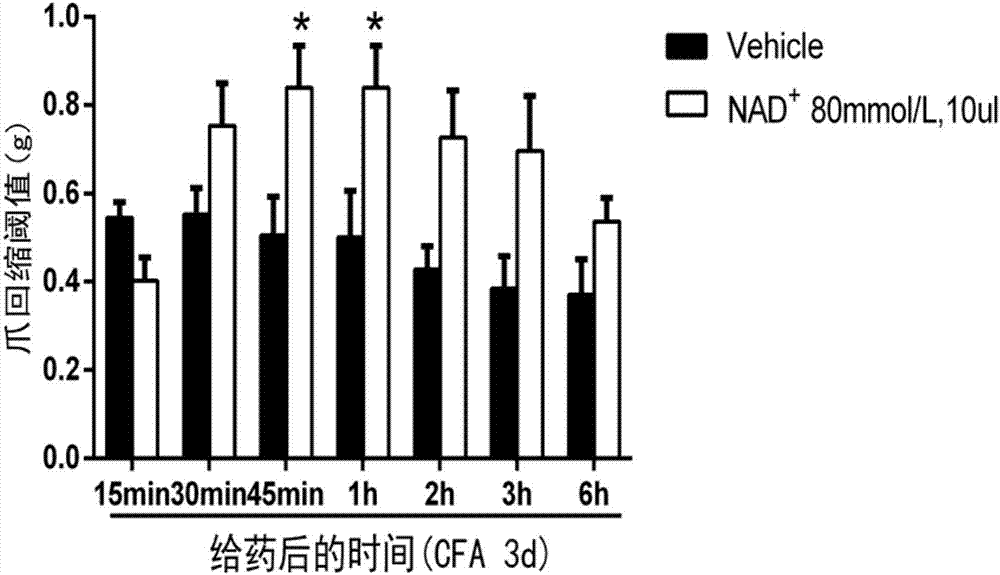
[0025] Take 18 ICR mice (male, about 20g), and divide them into two groups at random, namely the normal saline group (intrathecal injection, 10ul), the nicotinamide adenine dinucleotide group (80mmol / L, intrathecal injection, 10ul ). On the third day after injection of complete Freund's adjuvant into the soles of mice, physiological saline and nicotinamide adenine dinucleotide were administered intrathecally, and conventional von Frey fiber filaments were used to measure by up and down method, and the administration was determined respectively After 15min, 30min, 45min, 1h, 2h, 3h, 6h mechanical threshold, the results are as follows figure 2 As shown, compared with the normal saline group, the nicotinamide adenine dinucleotide group inhibited the inflammatory pain indu...
Embodiment 3
[0027] Nicotinamide adenine dinucleotide has an analgesic effect on inflammatory pain in mice induced by complete Freund's adjuvant, which will be further explained through pharmacodynamic experiments below.
[0028] Eighteen ICR mice (male, about 20 g) were taken and randomly divided into two groups. They were normal saline group (subcutaneous injection, 20ul) and nicotinamide adenine dinucleotide group (80mmol / L, subcutaneous injection, 20ul). On the third day after injection of complete Freund's adjuvant into the soles of the mice, physiological saline and 80 mmol / L nicotinamide adenine dinucleotide were subcutaneously administered to the soles of the mice, and measured by the up and down method using conventional vonFrey fiber filaments, respectively. Measuring the mechanical threshold at 15min, 30min, 45min, 1h, 2h, 3h, 6h after administration, the results are as follows image 3 As shown, compared with the saline group, the nicotinamide adenine dinucleotide group inhibi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com