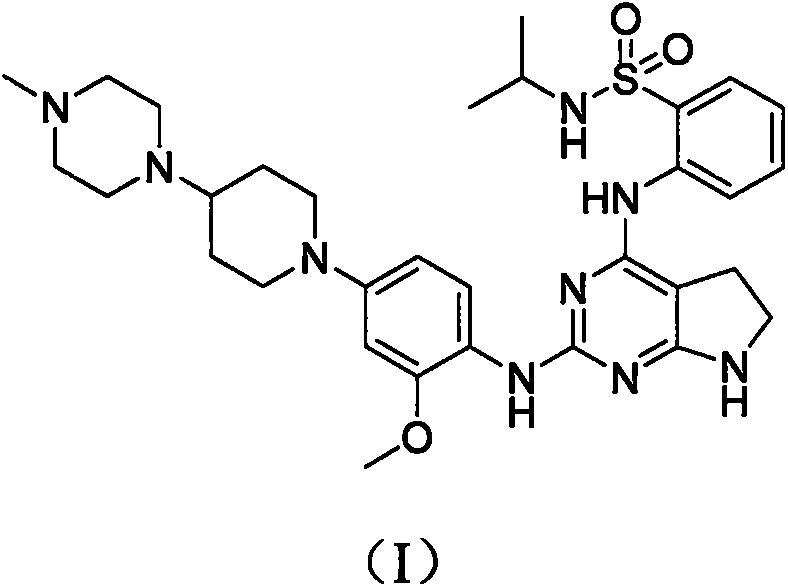
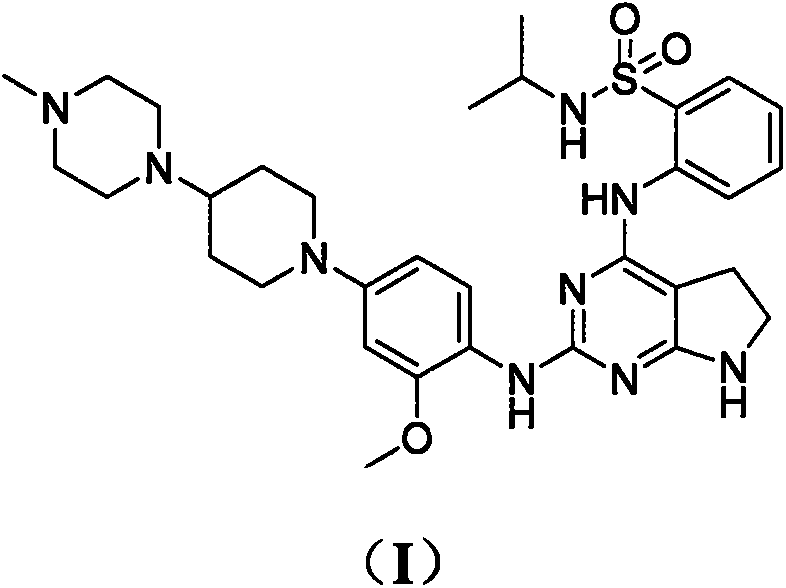
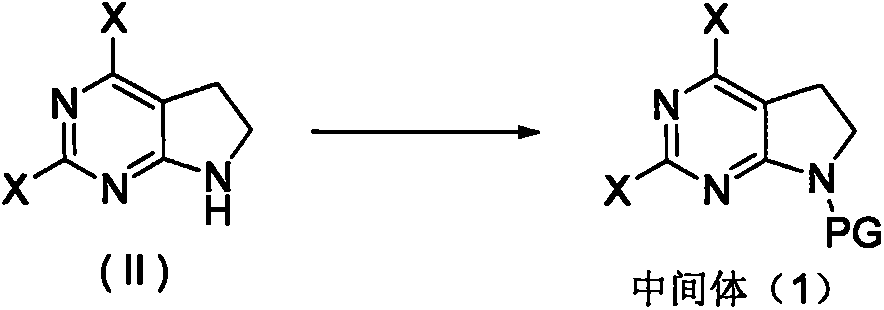
Preparation method of anaplastic lymphoma kinase inhibitor
A technology of an intermediate and a palladium catalyst is applied in the field of preparation of anaplastic lymphoma kinase inhibitors, and can solve the problems of long synthesis route, unsuitable for industrial production, complicated reaction operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Example 1: Synthesis of 7H-pyrrolo[2,3-d]pyrimidine-2,4-diol (Compound 2)
[0124]
[0125] Add compound 1 (21kg, 165mol), anhydrous sodium acetate (14.91kg, 181.8mol) and water (100L) into a 500L reactor, and raise the temperature to 70-75°C. Chloroacetaldehyde (49 kg, 40% aqueous solution, 249.7 mol) was added dropwise. After dropping, the temperature was raised to 90° C. to react for 3.5 hours, cooled to room temperature, and the reaction solution was centrifuged, washed with water, and dried to obtain 22.8 kg of brown powder as Compound 2 with a yield of 91.6%.
[0126] 1 H NMR (400MHz, DMSO-d 6): δ11.39 (1H, br), 11.05 (1H, br), 10.43 (1H, br), 6.53-6.54 (1H, d, J=5.2Hz), 6.18-6.19 (1H, d, J=5.2 Hz).
Embodiment 2
[0127] Example 2: Synthesis of 6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidine-2,4-diol (compound 3)
[0128]
[0129] Add compound 2 (20.5 kg, 135.6 mol) and trifluoroacetic acid (205 kg, 1797 mol) into a 500 L reactor from the upper port of the reactor, stir and mix well. The temperature was lowered to 0-5°C, and triethylsilane (37.9kg, 325mol) was added dropwise. After dropping, restore the temperature to 10-30°C and react overnight. Trifluoroacetic acid was evaporated under reduced pressure, 100kg of petroleum ether was added into the kettle, stirred, centrifuged, and the filter cake was dried to obtain 27.9kg of trifluoroacetic acid salt of compound 3 as a brown solid, with a yield of 77%.
[0130] 1 H NMR (400MHz, DMSO-d 6 ): δ11.07(1H, br), 10.48(1H, br), 10.06(1H, br), 3.49-3.53(2H, t, J=18Hz), 2.58-2.63(2H, t, J=18Hz) .
Embodiment 3
[0131] Example 3: Synthesis of 2,4-dichloro-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidine (compound 4)
[0132]
[0133] Add the trifluoroacetate (19.0kg, 71.1mol) of compound 3 obtained above into a 500L reactor, add phosphorus oxychloride (218kg, 1422mol), seal the above system and stir evenly, drop diiso Propylethylamine (32.2kg, 248mol) was added dropwise, heated to reflux overnight (about 12 hours). Phosphorus oxychloride was distilled off under reduced pressure, and a mixture of 150 L of water and 500 kg of ice was added to the residue with constant stirring to precipitate a solid, which was centrifugally filtered to obtain 4.05 kg of compound 4 as yellow crystals, with a yield of 30%.
[0134] 1 H NMR (400MHz, DMSO-d 6 ): δ6.45 (1H, br), 3.81-3.85 (2H, d, J=8.8Hz), 3.10-3.14 (2H, d, J=8.8Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com