Erysipelothrix rhusiopathiae antigen protein sbp and application
A technology of Erysipelothrix rhusiopathiae and antigenic protein, applied in the direction of bacterial antigenic components, applications, bacterial peptides, etc., can solve the problems of high cost, large protein molecular weight, and low conservation of SpaAN end, and achieve good protection effect and biological safety high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of Erysipelothrix rhusiopathiae antigenic protein (the present invention or claiming sbp protein):
[0026] The embodiment of the present invention takes prokaryotic expression as an example to illustrate the expression of the protein:
[0027] Construction of Escherichia coli BL21 / pET-28a-sbp, expression and purification of target protein
[0028] 1) Construction of expression vector
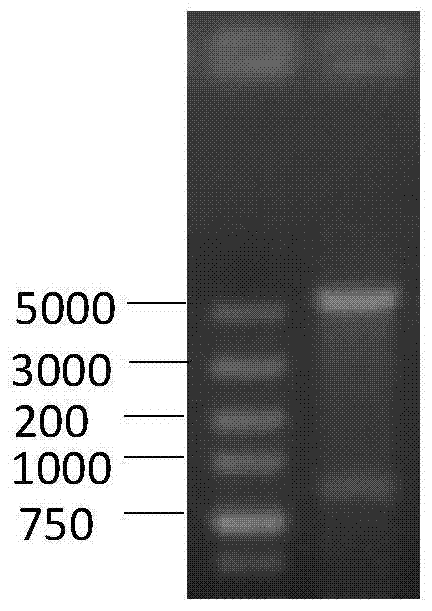
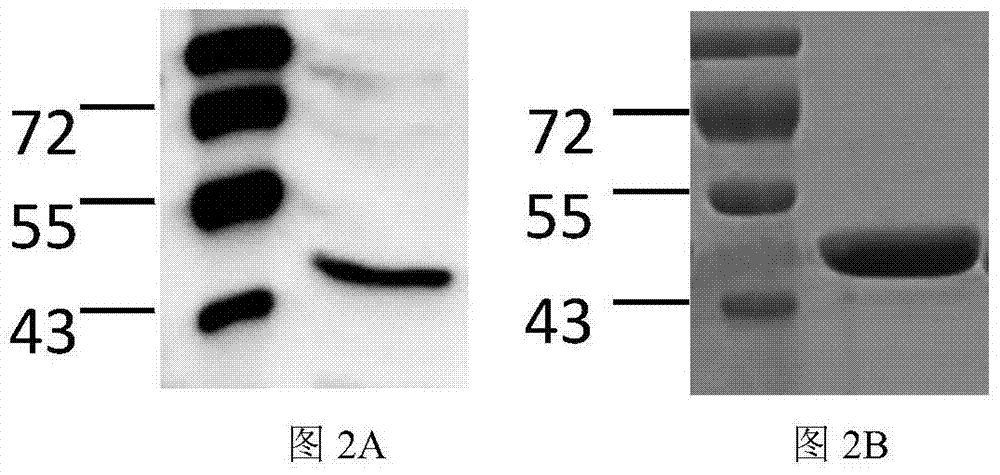
[0029] The sbp gene (sequence shown in SEQ ID NO.1) was inserted between the BamHI and XhoI restriction sites of pET-28a(+) according to conventional methods to construct the recombinant vector pET-28a-sbp. Transform into DH5a competent strains, pick positive clones to expand and culture, extract plasmids, use BamHI+XhoI to double-enzyme-digest the plasmids, and the foreign fragments of the expected size after digestion are correct recombinant plasmids. The results of double enzyme digestion identification of the recombinant plasmid were as follows: figure 1 As shown, the ...
Embodiment 2
[0034] Example 2: Subunit vaccine components and routine testing
[0035] Antigen component: sbp protein solution (expression, identification, purification and quantification of protein are as described in Example 1).
[0036] Dosage form: water-in-oil (W / O)
[0037] Adjuvant composition: white mineral oil (Marcol52)
[0038] Tween 80 (CRILLET4)
[0039] Span 80 (CRILL4)
[0040] Oil phase: heat white mineral oil to transparent and prepare with Span 80 at a volume ratio of 94:6.
[0041] Water phase: the sbp protein solution is composed of Tween 80, and Tween 80 accounts for 0.4% (volume ratio) of the water phase.
[0042] Vaccine emulsification: sbp protein is added to Tween 80 and mixed to form a water phase, and the water phase and oil phase are mixed and emulsified at a volume of 1:1.5. The final concentration of the antigenic protein sbp is 1mg / ml, which is used for the following experiments. The sbp protein vaccine provided by the present invention is also called sb...
Embodiment 3
[0047]Application of a kind of Erysipelothrix rhusiopathiae antigenic protein sbp in the preparation of porcine erysipelas subunit vaccine:
[0048] 1) Experimental scheme
[0049] Experimental animals: 30-day-old piglets (negative for Erysipelothrix rhusiopathiae antibody), purchased from Wuhan Tianzhong Qingcaohu Breeding Co., Ltd.
[0050] Attacking strain: Erysipelothrix rhusiopathiae SE38 strain (hereinafter referred to as SE38, Li Jingtao, Analysis of drug resistance of Erysipelothrix isolates in parts of Hubei, Breeding and Feed [J]. 2015.2, 11-14).
[0051] Experimental grouping: 30-day-old piglets were divided into 3 groups: immunization group 1 (sbp), immunization group 2 (sbp), adjuvant control group (adjuvant components described in Example 2 mixed with Tris-HCl), 6 pigs in each group . The immunization group 1 and the adjuvant control group were used for challenge, and the immunization group 2 was used to detect the antibody level, and the detection continued fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com