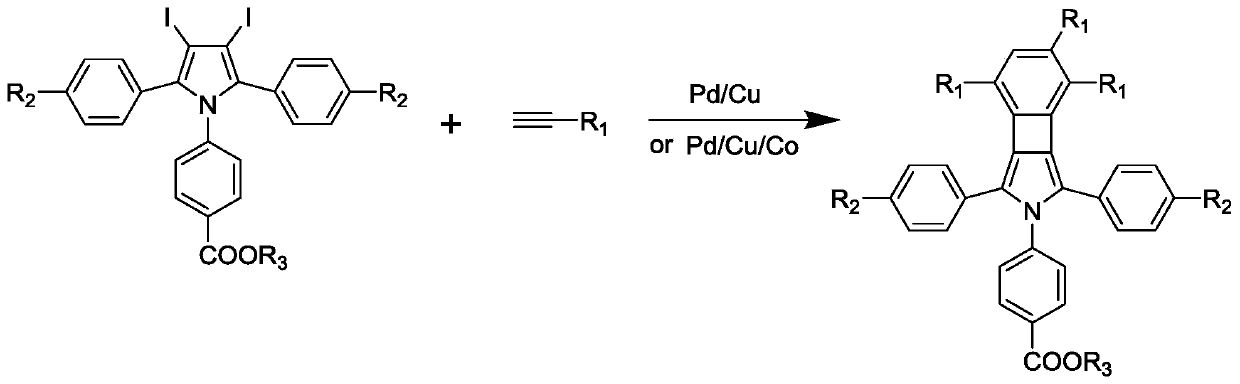
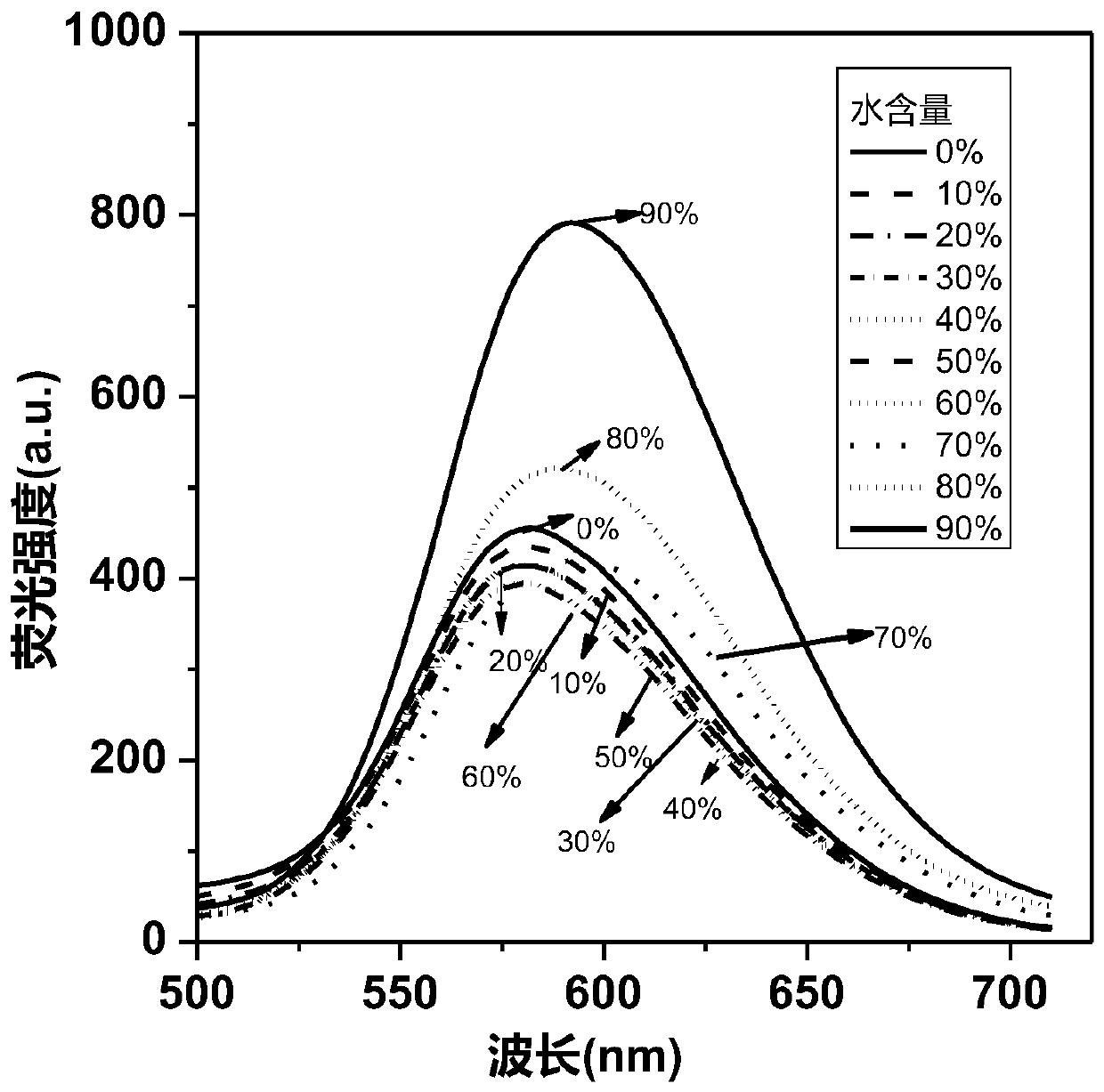
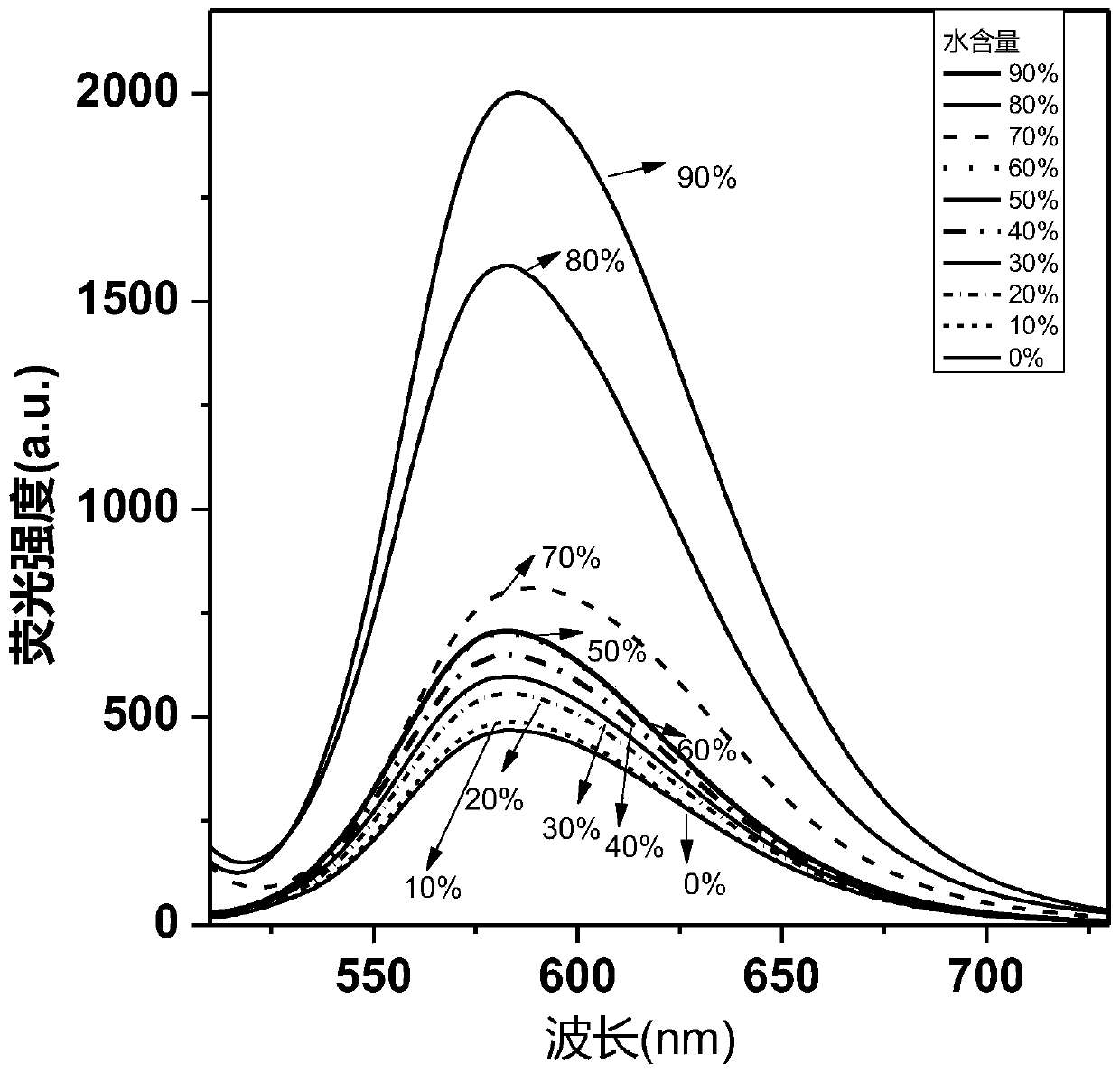
Benzocyclobutadienopyrrole derivatives with aee effect and their preparation
A technology for benzocyclobutadiene and pyrrole derivatives, which is applied in the field of organic synthesis, can solve problems such as limitation, and achieves the effects of cheap and easy-to-obtain raw materials and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Preparation of 1-p-benzoic acid ethyl-2,5-diphenyl-3,4-diiodopyrrole
[0046] (1.1) Add 1.2-diphenylethyne (2.0104g, 10.04mmol), ethyl p-aminobenzoate (6.6340g, 40.16mmol) and CuCl (0.0983g, 1.004mmol) into a 100mL polymerization tube, and vacuum Pass nitrogen three times; then, under the protection of nitrogen, first activate at 110°C for 30min, and then react at 140°C for 24h; Suction filtration after drying over anhydrous magnesium sulfate, spinning to obtain the crude product; use gel chromatography (dichloromethane:petroleum ether=1:2) to purify the crude product to obtain 2.0028g of white flocculent product, which is 1-Ethyl-p-benzoate-2,5-phenylpyrrole;
[0047] (1.2) 1-p-benzoic acid ethyl-2,5-phenylpyrrole (1.4846g, 3.90mmol), ICl (2.0082g, 12.83mmol) and NaHCO 3 (0.8668g, 10.56mmol) is added in the two-necked flask of 100mL, under nitrogen protection, add 30mL diethyl ether and react 24h under nitrogen protection; 5% hydrochloric acid aqueous solution w...
Embodiment 2
[0057] The 4-ethynyltoluene used in step (2) of Example 1 is replaced by 2-(4-phenylethynyl ether)-1,3-dioxolane with a molar number of 2 mmol, and other steps and parameters are the same as in Example 1 Same, that is, benzocyclobutadienopyrrole derivatives are obtained.
[0058] The product prepared in this implementation is characterized, and the data of its proton nuclear magnetic spectrum and mass spectrum are as follows:
[0059] 1 H NMR (400MHz, CDCl 3 )δ7.74(dd, J=8.3,6.6Hz,2H),7.66(d,J=8.2Hz,1H),7.51(d,J=8.0Hz,2H),7.44(d,J=8.2Hz, 1H),7.25–7.20(m,1H),7.19(d,J=3.4Hz,3H),7.17–7.10(m,3H),7.08(d,J=6.6Hz,1H),7.03(dd,J =13.1,5.4Hz,2H),6.87(ddd,J=21.0,13.9,7.8Hz,6H),6.77(t,J=7.2Hz,2H),6.62(dd,J=17.7,9.9Hz,2H) ,6.46(d,J=7.3Hz,1H),5.85(d,J=17.0Hz,1H),5.77–5.70(m,1H),5.62(s,1H),4.30(qd,J=7.1,3.4 Hz, 2H), 4.18–3.95(m, 12H), 1.33(td, J=7.1, 3.1Hz, 3H;
[0060] MS (ESI + ):Molecular formula C 58 h 48 o 8 N, m / z: calculated value [M+H] + =886.3374, test value [M+H] + =886...
Embodiment 3
[0065] The 4-ethynyltoluene used in the step (2) of Example 1 was replaced by phenylacetylene of an equimolar number, and the reaction at 100°C for 24h in the step (2) of Example 1 was replaced by the reaction at 60°C for 15h, The other steps and parameters were the same as in Example 1 to obtain benzocyclobutadienopyrrole derivatives.
[0066] The product prepared in this implementation is characterized, and the data of its proton nuclear magnetic spectrum and mass spectrum are as follows:
[0067] 1 H NMR (400MHz, CDCl 3 )δ7.79–7.64(m,3H),7.50(dd,J=20.7,7.5Hz,1H),7.43–7.29(m,3H),7.22–6.44(m,23H),4.30(q,J= 7.0Hz, 2H), 1.34(t, J=7.1Hz, 3H);
[0068] MS(MALDI-TOF): Molecular Formula C 49 h 35 NO 2 , m / z: calculated value [M] = 669.2668, test value [M] = 669.2685;
[0069] According to the characterization results of proton nuclear magnetic spectrum and mass spectrum, the structural formula of the benzocyclobutadienopyrrole derivative prepared in this embodiment is as fol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com