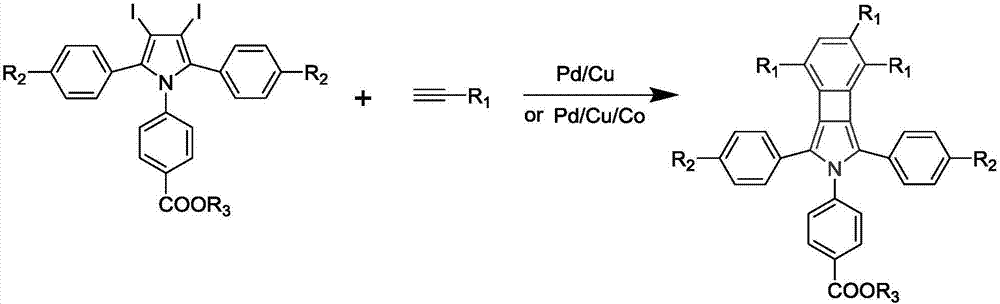
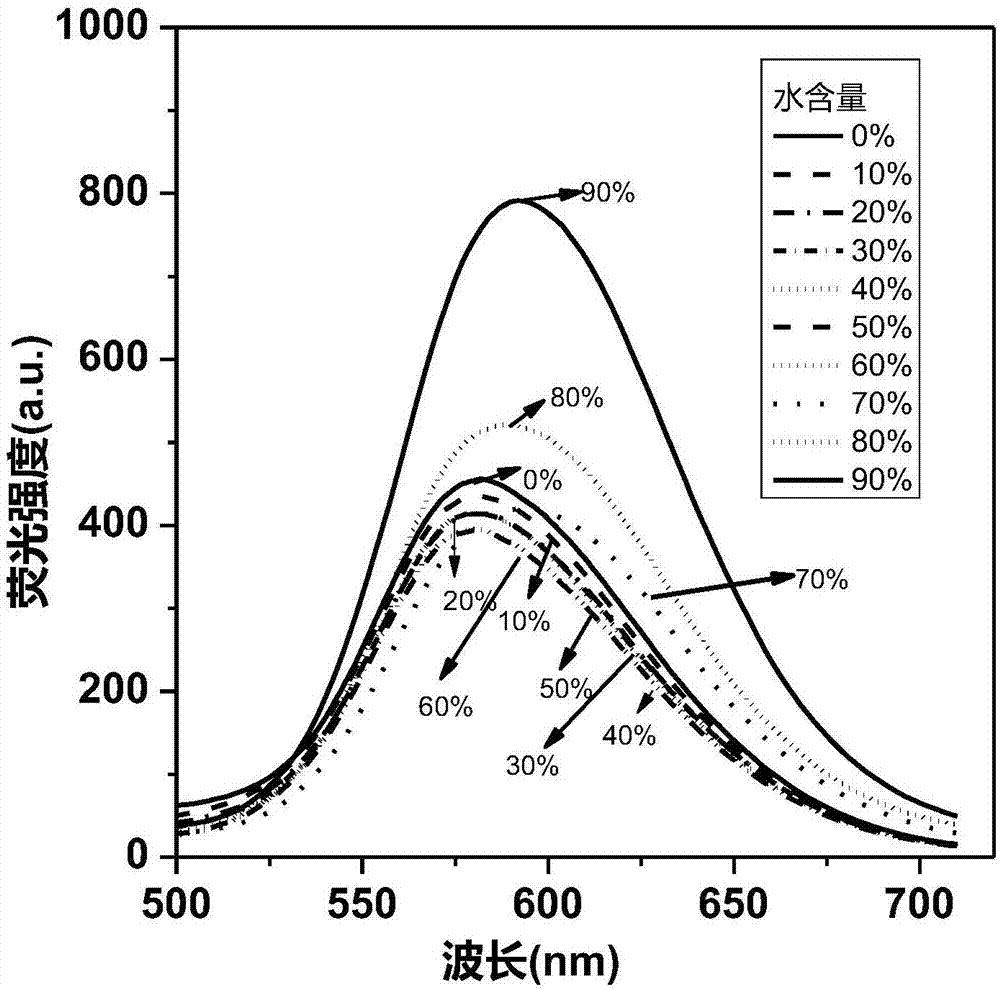
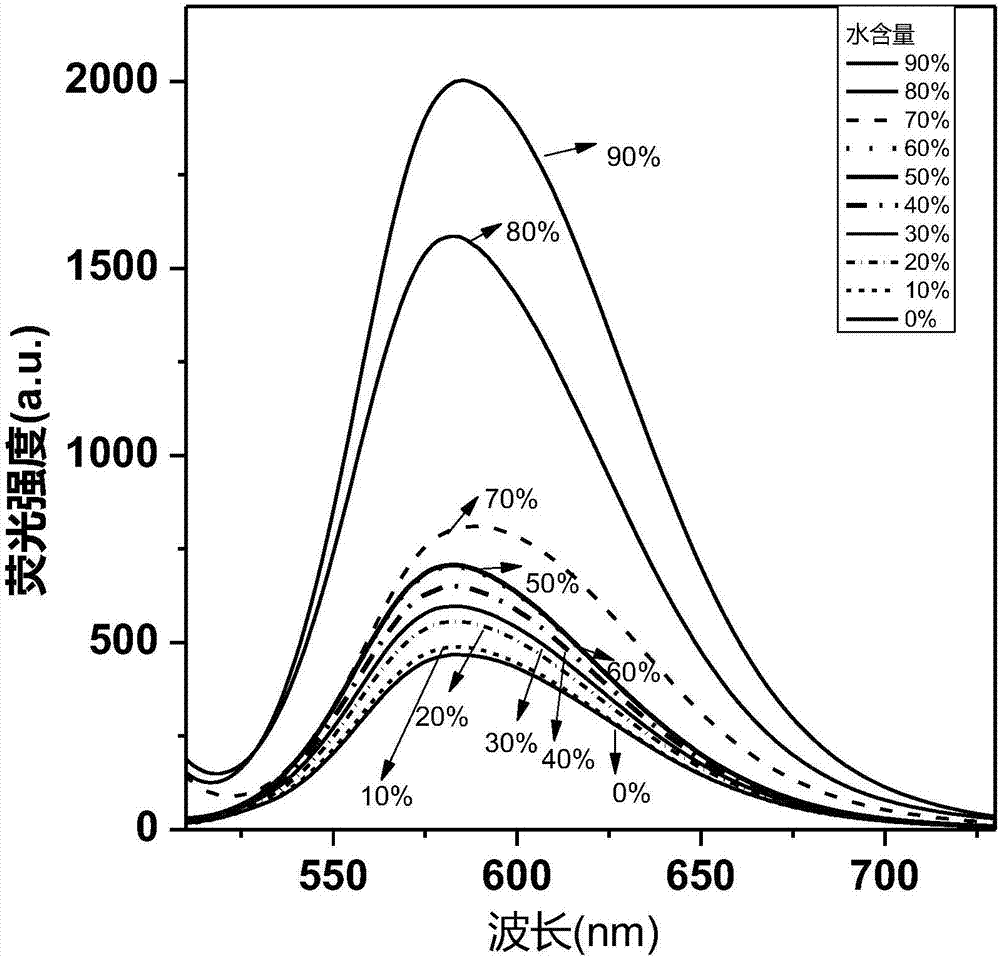
Cyclobutadiene benzopyrrole derivative with AEE effect and preparation thereof
A technology for benzocyclobutadiene and pyrrole derivatives is applied in the fields of benzocyclobutadiene and pyrrole derivatives and their preparation, and in the field of fluorescent compounds. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Preparation of 1-p-benzoic acid ethyl-2,5-diphenyl-3,4-diiodopyrrole
[0046] (1.1) Add 1.2-diphenylethyne (2.0104g, 10.04mmol), ethyl p-aminobenzoate (6.6340g, 40.16mmol) and CuCl (0.0983g, 1.004mmol) into a 100mL polymerization tube, and vacuum Pass nitrogen three times; then, under the protection of nitrogen, first activate at 110°C for 30min, and then react at 140°C for 24h; Suction filtration after drying over anhydrous magnesium sulfate, spinning to obtain the crude product; use gel chromatography (dichloromethane:petroleum ether=1:2) to purify the crude product to obtain 2.0028g of white flocculent product, which is 1-Ethyl-p-benzoate-2,5-phenylpyrrole;
[0047] (1.2) 1-p-benzoic acid ethyl-2,5-phenylpyrrole (1.4846g, 3.90mmol), ICl (2.0082g, 12.83mmol) and NaHCO 3 (0.8668g, 10.56mmol) is added in the two-necked flask of 100mL, under nitrogen protection, add 30mL diethyl ether and react 24h under nitrogen protection; 5% hydrochloric acid aqueous solution w...
Embodiment 2
[0057] The 4-ethynyltoluene used in step (2) of Example 1 is replaced by 2-(4-phenylethynyl ether)-1,3-dioxolane with a molar number of 2 mmol, and other steps and parameters are the same as in Example 1 Same, that is, benzocyclobutadienopyrrole derivatives are obtained.
[0058] The product prepared in this implementation is characterized, and the data of its proton nuclear magnetic spectrum and mass spectrum are as follows:
[0059] 1 H NMR (400MHz, CDCl 3 )δ7.74(dd, J=8.3,6.6Hz,2H),7.66(d,J=8.2Hz,1H),7.51(d,J=8.0Hz,2H),7.44(d,J=8.2Hz, 1H),7.25–7.20(m,1H),7.19(d,J=3.4Hz,3H),7.17–7.10(m,3H),7.08(d,J=6.6Hz,1H),7.03(dd,J =13.1,5.4Hz,2H),6.87(ddd,J=21.0,13.9,7.8Hz,6H),6.77(t,J=7.2Hz,2H),6.62(dd,J=17.7,9.9Hz,2H) ,6.46(d,J=7.3Hz,1H),5.85(d,J=17.0Hz,1H),5.77–5.70(m,1H),5.62(s,1H),4.30(qd,J=7.1,3.4 Hz, 2H), 4.18–3.95(m, 12H), 1.33(td, J=7.1, 3.1Hz, 3H;
[0060] MS (ESI + ):Molecular formula C 58 h 48 o 8 N, m / z: calculated value [M+H] + =886.3374, test value [M+H] + =886...
Embodiment 3
[0065] The 4-ethynyltoluene used in the step (2) of Example 1 was replaced by phenylacetylene of an equimolar number, and the reaction at 100°C for 24h in the step (2) of Example 1 was replaced by the reaction at 60°C for 15h, The other steps and parameters were the same as in Example 1 to obtain benzocyclobutadienopyrrole derivatives.
[0066] The product prepared in this implementation is characterized, and the data of its proton nuclear magnetic spectrum and mass spectrum are as follows:
[0067] 1 H NMR (400MHz, CDCl 3 )δ7.79–7.64(m,3H),7.50(dd,J=20.7,7.5Hz,1H),7.43–7.29(m,3H),7.22–6.44(m,23H),4.30(q,J= 7.0Hz, 2H), 1.34(t, J=7.1Hz, 3H);
[0068] MS(MALDI-TOF): Molecular Formula C 49 h 35 NO 2 , m / z: calculated value [M] = 669.2668, test value [M] = 669.2685;
[0069] According to the characterization results of proton nuclear magnetic spectrum and mass spectrum, the structural formula of the benzocyclobutadienopyrrole derivative prepared in this embodiment is as fol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com