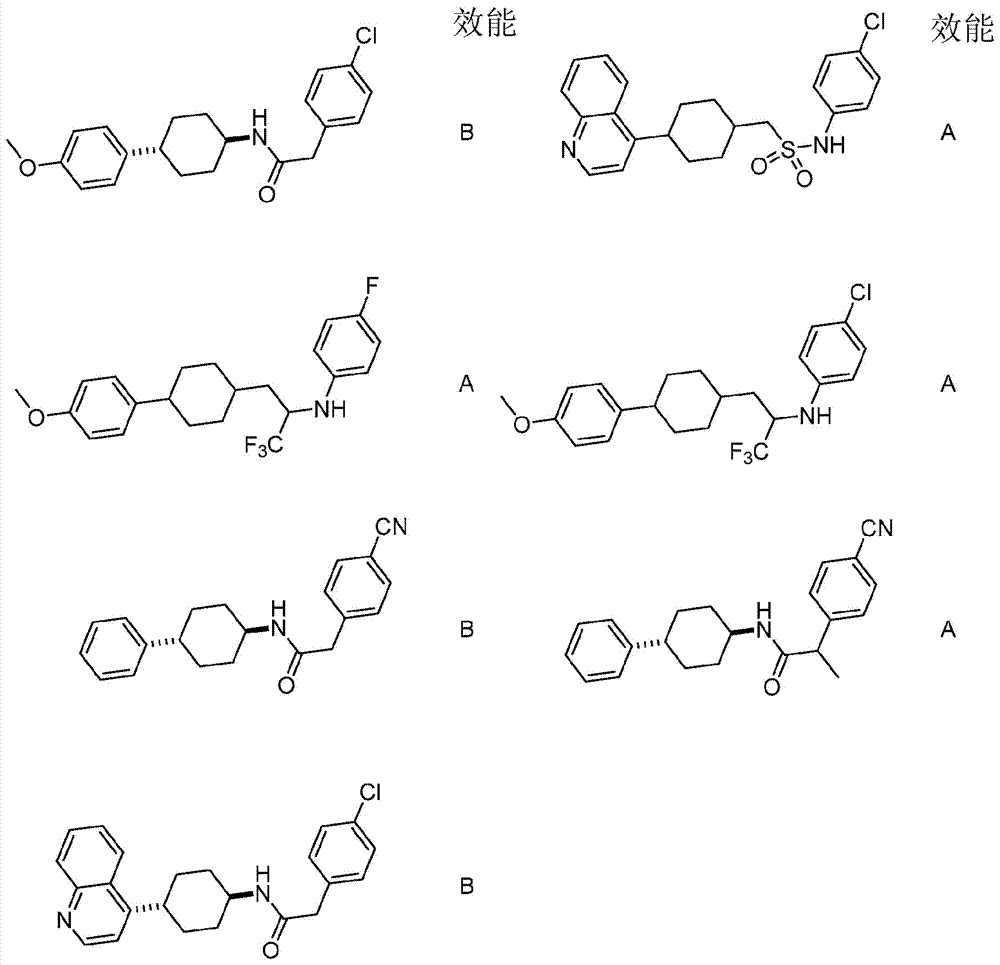
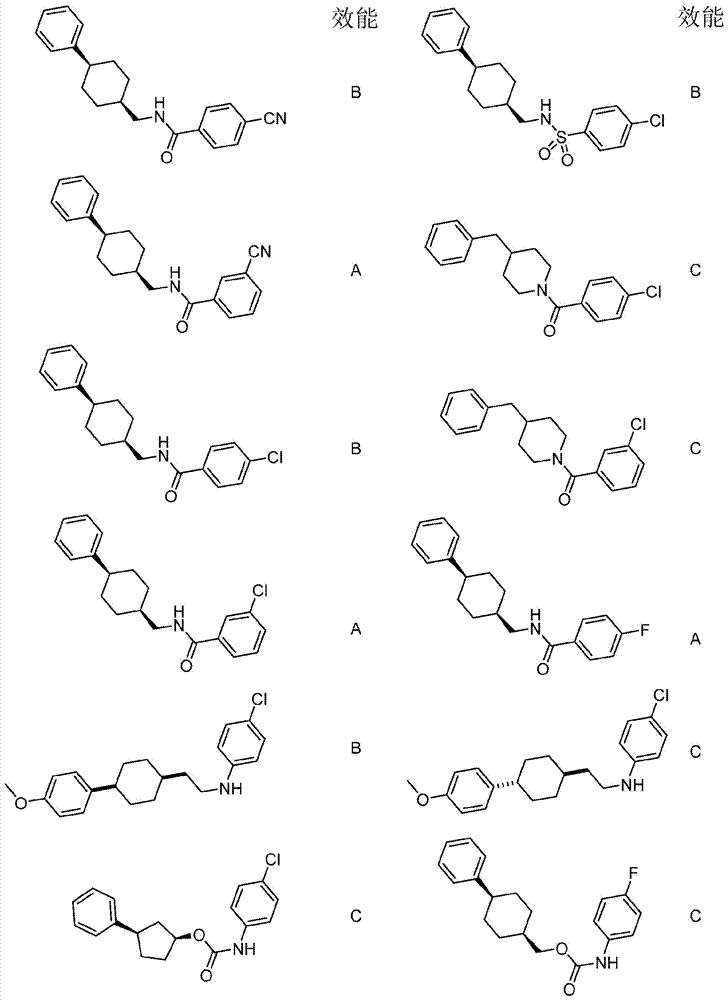
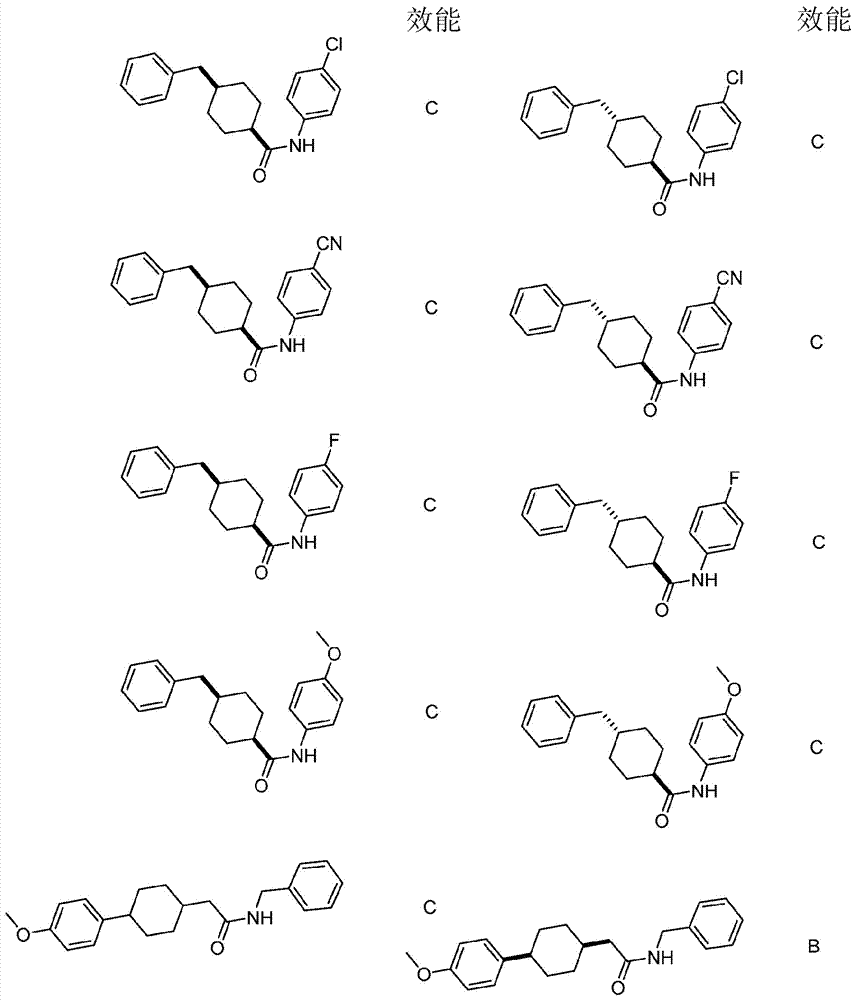
Immunoregulatory agents
A technology of solvates and compounds, applied in the field of compounds that regulate oxidoreductase indoleamine 2,3-dioxygenase, can solve problems such as different functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0364] cis-4-cyano-N-((4-phenylcyclohexyl)methyl)benzamide
[0365]
[0366] Using general procedure B, use a mixture containing cis-(4-phenylcyclohexyl)methanamine (19 mg, 0.1 mmol), 4-cyanobenzoyl chloride (17 mg, 0.1 mmol) and NEt 3 (51 mg, 0.5 mmol) CH 2 Cl 2 (1 mL) was prepared. Purification using silica gel chromatography (10% to 30% EtOAc / hexanes) gave the desired product as a white solid. 1 H NMR (400MHz; CDCl 3 ):δ7.89-7.86(m,2H),7.74-7.71(m,2H),7.32-7.17(m,5H),6.32-6.30(m,1H),3.58(dd,J=7.7,6.0Hz ,2H),2.66-2.59(m,1H),2.06-2.00(m,1H),1.80-1.69(m,8H). m / z 319.3 (M+H + ).
Embodiment 2
[0368] cis-3-cyano-N-((4-phenylcyclohexyl)methyl)benzamide
[0369]
[0370] Using general procedure B, use a mixture containing cis-(4-phenylcyclohexyl)methanamine (19 mg, 0.1 mmol), 3-cyanobenzoyl chloride (17 mg, 0.1 mmol) and NEt 3 (51 mg, 0.5 mmol) CH 2 Cl 2 (1 mL) was prepared. Purification using silica gel chromatography (10% to 30% EtOAc / hexanes) gave the desired product as a white solid. 1 H NMR (400MHz; CDCl 3 ):δ8.09-8.08(m,1H),8.04(dt,J=7.9,1.5Hz,1H),7.76(dt,J=7.7,1.3Hz,1H),7.56(t,J=7.8Hz, 1H), 7.32-7.17(m, 5H), 6.49-6.46(m, 1H), 3.58(dd, J=7.7, 6.0Hz, 2H), 2.66-2.59(m, 1H), 2.07-2.01(m, 1H), 1.80-1.68 (m, 8H). m / z 319.2 (M+H + ).
Embodiment 3
[0372] cis-4-chloro-N-((4-phenylcyclohexyl)methyl)benzamide
[0373]
[0374] Using general procedure B, use a mixture containing cis-(4-phenylcyclohexyl)methanamine (19 mg, 0.1 mmol), 4-chlorobenzoyl chloride (19 mg, 0.1 mmol) and NEt 3 (51 mg, 0.5 mmol) CH 2 Cl 2(1 mL) was prepared. Purification using silica gel chromatography (0% to 20% EtOAc / Hexanes) gave the desired product as a white solid. 1 H NMR (400MHz; CDCl 3 ):δ7.73-7.69(m,2H),7.41-7.38(m,2H),7.31-7.23(m,4H),7.20-7.16(m,1H),3.55(dd,J=7.7,5.9Hz ,2H), 2.63-2.59(m,1H), 2.04-1.98(m,1H), 1.79-1.67(m,8H). m / z 328.2 (M+H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com