Synthesis method of functionally-substituted dicyanoethylene compounds
A technology of dicyanoethylene and synthesis method, which is applied in the field of chemistry, can solve problems such as complex operation and environmental hazards, and achieve the effects of simple operation, environmental friendliness and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: 3,4-dibromo-2, the synthesis of 5-dinitrothiophene
[0074]
[0075] 5g of 3,4-dibromothiophene and 10ml of mixed acid (concentrated H 2 SO 4 and concentrated HNO 3 Volume ratio 1:1) was reacted in 5ml chloroform, the reaction temperature was 50°C, the reaction time was 4h, and 6.9g of the product was obtained, the mixed acid was removed, and recrystallized with dichloromethane and petroleum ether (volume ratio 1:4), the yield was 90 %. 13 C NMR (100 MHz, CDCl 3 ) δ 147.68, 117.51.
Embodiment 2
[0076] Example 2: Synthesis of 2,5-dinitro-3,4-diphenylthiophene
[0077]
[0078] Weigh 0.5g (1.52mmol) of the compound 3,4-dibromo-2,5-dinitrothiophene, 0.75g (6.1mmol) of phenylboronic acid, 0.4g (6.8mmol) of KF and four Put 240 mg of triphenylphosphine palladium in a reaction flask, add 24 ml of toluene, 10 ml of water, and 5 ml of ethanol, and ventilate to make the reaction system an oxygen-free environment. Adjust the temperature to 110°C and react for 24h. After the reaction was completed, it was extracted with ethyl acetate, dried over magnesium sulfate, and petroleum ether and dichloromethane (volume ratio 4:1) were used as eluents to pass through the column. After weighing, 0.36 g of product 2a was obtained with a yield of 72%.
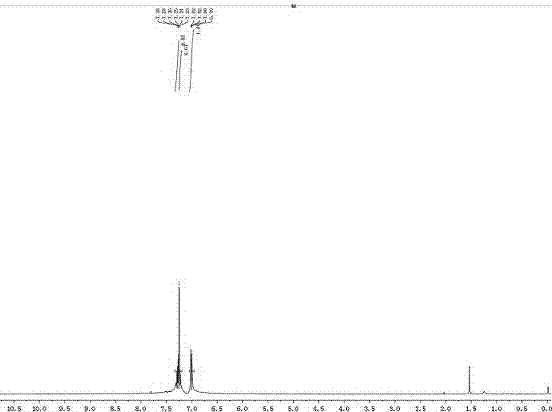
[0079] Product 2a 1 H NMR as figure 1 shown. 1 H NMR (400 MHz, DMSO) δ 7.32-7.23 (m, 2H), 7.20-7.13 (m, 1H).
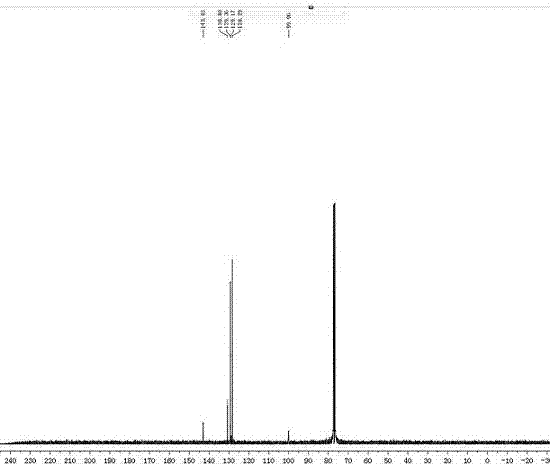
[0080] Product 2a 13 C NMR such as figure 2 shown. 13 C NMR (100MHz, CDCl 3 ) δ 147.47, 143.07, 130.81, 129.33, 129....
Embodiment 3
[0081] Example 3: Synthesis of 2,5-dinitro-3,4-di-p-tolylthiophene
[0082]
[0083] Weigh 0.5g (1.52mmol) of the compound 3,4-dibromo-2,5-dinitrothiophene obtained in Example 1, 0.83g (6.1mmol) of p-tolylboronic acid, and 0.4g (6.8mmol) of KF Put 240 mg of tetraphenylphosphine palladium in a reaction flask, add 24 ml of toluene, 10 ml of water and 5 ml of ethanol, and ventilate to make the reaction system an oxygen-free environment. Adjust the temperature to 110°C and react for 24h. After the reaction was completed, it was extracted with ethyl acetate, dried over magnesium sulfate, and petroleum ether and dichloromethane (volume ratio 4:1) were used as eluents to pass through the column. After weighing, 0.38 g of product 2b was obtained with a yield of 70%.
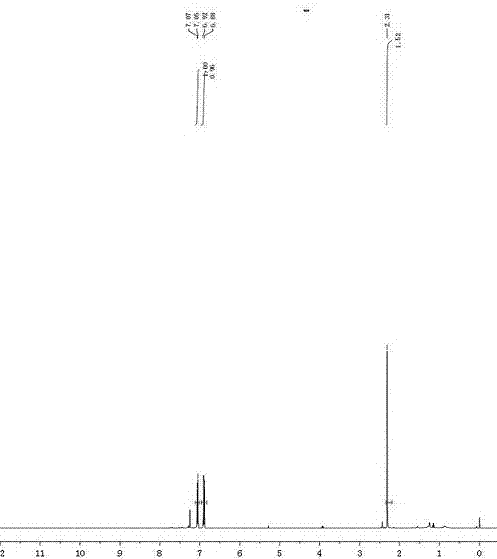
[0084] Product 2b 1 H NMR as image 3 shown. 1 H NMR (400 MHz, CDCl 3 ) δ 7.06 (d, J=7.9Hz, 2H), 6.89 (d, J=8.0Hz, 2H), 2.31 (s, 3H).
[0085] Product 2b 13 C NMR such as Figure 4 shown. 13 C NMR (100 MHz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com