Preparation method of high-purity cis-3,5-dimethylpiperidine
A technology of dimethylpiperidine and lutidine, which is applied in the field of preparation of high-purity cis-3,5-dimethylpiperidine, can solve problems such as the complex ratio of cis, and achieve high commercial value and process Simple, high product purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
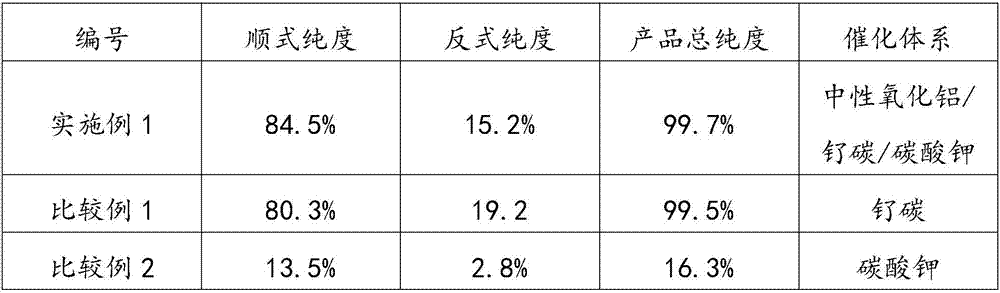
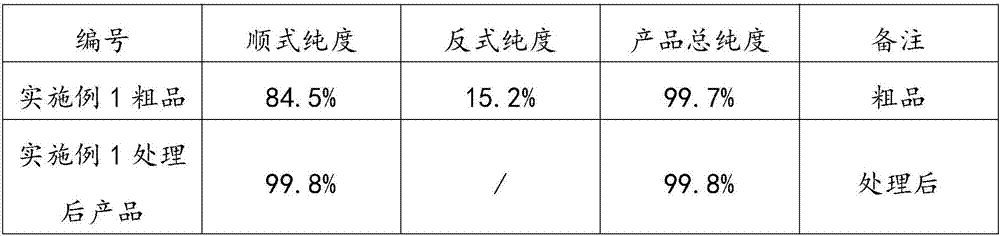
[0039] Add 645.0g of 3,5-lutidine, 77.4g of neutral alumina, 12.9g of potassium carbonate, and 25.8g of ruthenium on carbon (5% effective content) into a 2L autoclave. Close the autoclave, replace with nitrogen 6 times, and replace with hydrogen 6 times. After charging and heating to a hydrogen pressure of 9.0MPa and 150°C, the hydrogen pressure will drop significantly during the reaction process. The heat-preservation and pressure-holding reaction lasts for 5.0 hours, and the complete reaction of 3,5-lutidine is detected. The reaction crude product is obtained by suction filtration. The rate is 96.8%. Gas chromatography analysis showed that the purity of cis-3,5-dimethylpiperidine was 84.5%, and the purity of trans-3,5-dimethylpiperidine was 15.2%.
[0040] The 2L three-necked bottle is equipped with a mechanical stirrer, a thermometer, and a drying tube. Add the above reaction crude product, 340.0g n-hexane, and 35.0g absolute ethanol into a 2L three-necked flask. After r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com