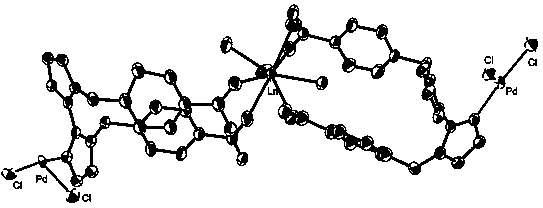
PD/ln heterometallic organic framework and its preparation method and application
An organic framework and heterogeneous metal technology, applied in the field of chemical functional materials and their preparation, can solve the problems of difficult recovery and reuse of palladium, waste, polluted products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: Catalyst [H 3 O]n[SmPd 2 [L] 2 Cl 4 ]n or [H 3 O]n[EuPd 2 [L] 2 Cl 4 ]n or [H 3 O]n[TbPd 2 [L] 2 Cl 4 ]n or [H 3 O]n[DyPd 2 [L] 2 Cl 4 ]n preparation
[0038] (1) 0.145 mmol 1,1'-bis(p-carboxybenzyl)-2,2'-bis-imidazole ligand, 0.09 mmol Sm(NO 3 ) 3 ·6H 2 O or Eu(NO 3 ) 3 ·6H 2 O or Tb(NO 3 ) 3 ·6H 2 O or Dy(NO 3 ) 3 ·6H 2 O, 0.15 mmol K 2 PdCl 4 , add 9mLH 2 O and 2mL of acetonitrile mixed solution, stirred at room temperature for 15min, a light yellow turbid solution appeared. The above solution was sealed in a reaction kettle with a 23mL polytetrafluoroethylene liner, kept at a temperature of 100°C for 72h, and cooled to room temperature. Yellow blocky crystals were obtained with a yield of 71%. The molecular formula is: [H 3 O]n[SmPd 2 [L] 2 Cl 4 ]n or [H 3 O]n[EuPd 2 [L] 2 Cl 4 ]n or [H 3 O]n[TbPd 2 [L] 2 Cl 4 ]n or [H 3 O]n[DyPd 2 [L] 2 Cl 4 ] n.
[0039] (2) 0.145 mmol 1,1'-di(p-carboxybenzyl)-2,2'-bis-im...
Embodiment 2
[0048] Example 2: Pd / Ln heterometallic organic framework [H 3 O]n[SmPd 2 [L] 2 Cl 4 ]n or [H 3 O]n[EuPd 2 [L] 2 Cl 4 ]n or [H 3 O]n[TbPd 2 [L] 2 Cl 4 ]n or [H 3 O]n[DyPd 2 [L] 2 Cl 4 Catalytic Activity of ]n on Suzuki C-C Coupling Reaction
[0049] (1) The yellow bulk crystal obtained in Example 1 [H 3 O]n[SmPd 2 [L] 2 Cl 4 ]n was used to verify its catalytic activity in the Suzuki C–C coupling reaction of aryl halides and phenylboronic acid. Specific operation method: 1 mmol aryl halide (chlorobenzene, bromobenzene or iodobenzene), 1.2 mmol phenylboronic acid, 2 mmol potassium carbonate and 0.4 mol% [H 3 O]n[SmPd 2 [L] 2 Cl 4 ]n into a round-bottomed flask, 6mL of deionized water as the reaction solvent, magnetically stirred at a temperature of 80 °C for 6 h, the solution after the reaction was extracted with ethyl acetate, and dried with anhydrous sodium sulfate, and then the organic solvent was removed Obtain final product, productive rate is measured...
Embodiment 3
[0061] Example 3: Pd / Ln heterometallic organic framework [H 3 O]n[SmPd 2 [L] 2 Cl 4 ]n or [H 3 O]n[EuPd 2 [L] 2 Cl 4 ]n or [H 3 O]n[TbPd 2 [L] 2 Cl 4 ]n or [H 3 O]n[DyPd 2 [L] 2 Cl 4 ]n Catalytic activity for recycling in Suzuki C–C coupling reactions.
[0062] (1) Deionized water as the reaction solvent: After the Suzuki C-C coupling reaction in Example 2 (1)~(4) is completed, follow the instructions attached Figure 5 The method shown is to adjust the appropriate pH with the catalyst [H in the above reaction 3 O]n[SmPd 2 [L] 2 Cl 4 ]n or [H 3 O]n[EuPd 2 [L] 2 Cl 4 ]n or [H 3 O]n[TbPd 2 [L] 2 Cl 4 ]n or [H 3 O]n[DyPd 2 [L] 2 Cl 4 ]n separation, and put it into the Suzuki C-C coupling reaction of aryl halide and phenylboronic acid to verify its circular catalytic activity. Specific operation method: 1 mmol aryl halide (chlorobenzene, bromobenzene or iodobenzene), 1.2 mmol phenylboronic acid, 2 mmol potassium carbonate, and 0.4 mol% catalyst [H 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com