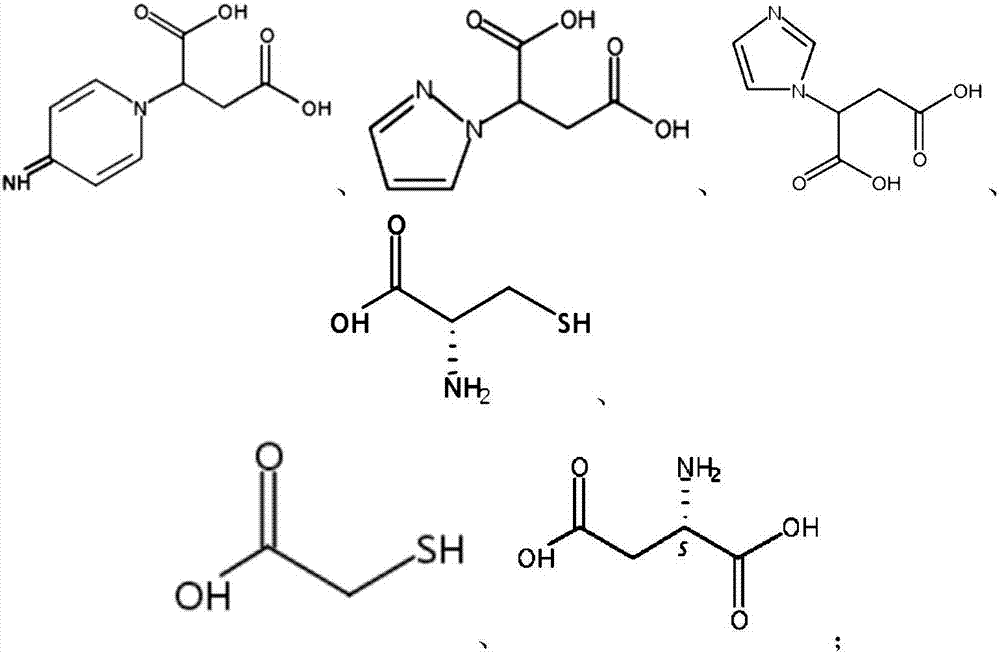
1,3-propanediol and preparation method thereof
A technology for esterification and reactant, which is applied in the field of solid esterification and its melt condensation preparation based on the difference in the reactivity of the multifunctional groups of the reactants, can solve the problems of environmental pollution in the preparation process, difficulty in storage and use, and low content of functional elements, etc. Achieve the effect of overcoming easy migration, beneficial to environmental protection and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
[0044] A kind of preparation method of esterification compound, the steps are as follows:
[0045] 1) The side group phosphorus-containing dibasic acid [(6-oxo-6H-dibenzo[c,e][1,2]oxaphosphorin-6-yl)methyl]succinic acid (DDP ) and pentaerythritol are mixed with a molar ratio of 4:1, under the conditions of inert gas nitrogen protection and mechanical stirring, the melt esterification reaction is carried out, the stirring speed of the mechanical stirrer is 300rpm, the temperature of the melt esterification reaction is 180 ℃, and the reaction time is After 1h, the product was collected after the reaction, dissolved, filtered and dried to obtain DAPER whose end group was carboxyl; the reaction equation is as follows:
[0046]
[0047] 2) Mix phenylphosphonic acid and glycol with a molar ratio of 1:1, add catalyst 4-methylbenzenesulfonic acid, the molar ratio of phenylphosphonic acid and catalyst is 1:0.01, under inert gas nitrogen protection and The melt condensation reaction...
Embodiment 4~6
[0057] A kind of preparation method of esterification compound, the steps are as follows:
[0058] 1) Mix side group nitrogen-containing dibasic acid and pentaerythritol in a molar ratio of 4:1, and carry out melt esterification reaction under the conditions of inert gas nitrogen protection and mechanical stirring, the stirring speed of mechanical stirring is 310rpm, melt esterification reaction The temperature is 180°C, and the reaction time is 2 hours. After the reaction, the product is collected, dissolved, filtered and dried to obtain DAPER whose end group is a carboxyl group; the reaction equation is as follows:
[0059]
[0060] 2) Mix phenylphosphonic acid and glycol with a molar ratio of 1:2, add catalyst 4-methylbenzenesulfonic acid, the molar ratio of phenylphosphonic acid and catalyst is 1:0.01, under inert gas nitrogen protection and The melt condensation reaction was carried out under the condition of mechanical stirring, the stirring speed of the mechanical st...
Embodiment 7~9
[0071] A kind of preparation method of esterification compound, the steps are as follows:
[0072] 1) Mix side group nitrogen-containing dibasic acid and pentaerythritol in a molar ratio of 4:1, and carry out melt esterification reaction under the conditions of inert gas nitrogen protection and mechanical stirring. The stirring speed of mechanical stirring is 340rpm, and melt esterification reaction The temperature is 182°C, and the reaction time is 3 hours. After the reaction, the product is collected, dissolved, filtered and dried to obtain DAPER whose terminal group is a carboxyl group; the reaction equation is as follows:
[0073]
[0074] 2) Methylphosphonic acid and dibasic alcohol are mixed with the molar ratio of 1:1, add catalyst 4-methylbenzenesulfonic acid, the molar ratio of methylphosphonic acid and catalyst is 1:0.01, under inert gas nitrogen protection and The melt condensation reaction is carried out under the condition of mechanical stirring, the stirring s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com