A kind of amino polyol fumagillin and its synthesis method and application
A technology of fumagillin and synthesis method, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, drug combinations, etc., can solve problems such as poor water solubility, achieve reduced toxicity, non-toxic product solubility, and high product solubility Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
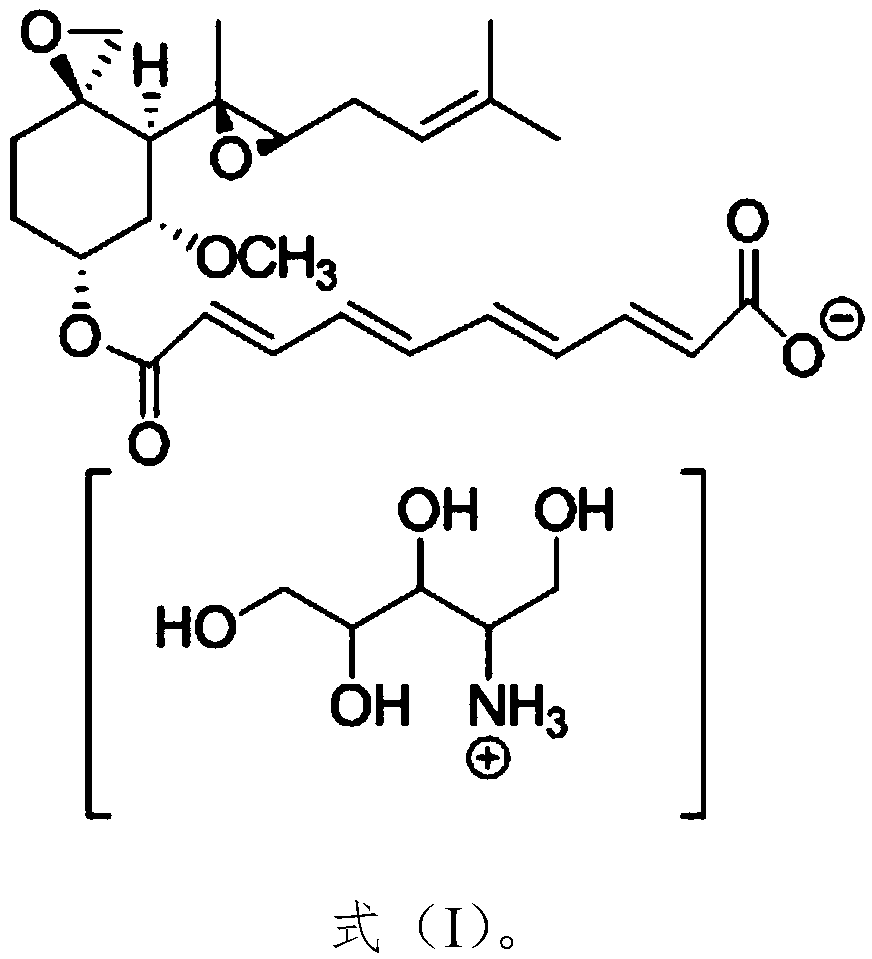
[0028] The present embodiment provides amino polyol fumagillin as shown in formula I:
[0029]
[0030] Its NMR spectrum is shown in figure 1 .
Embodiment 2
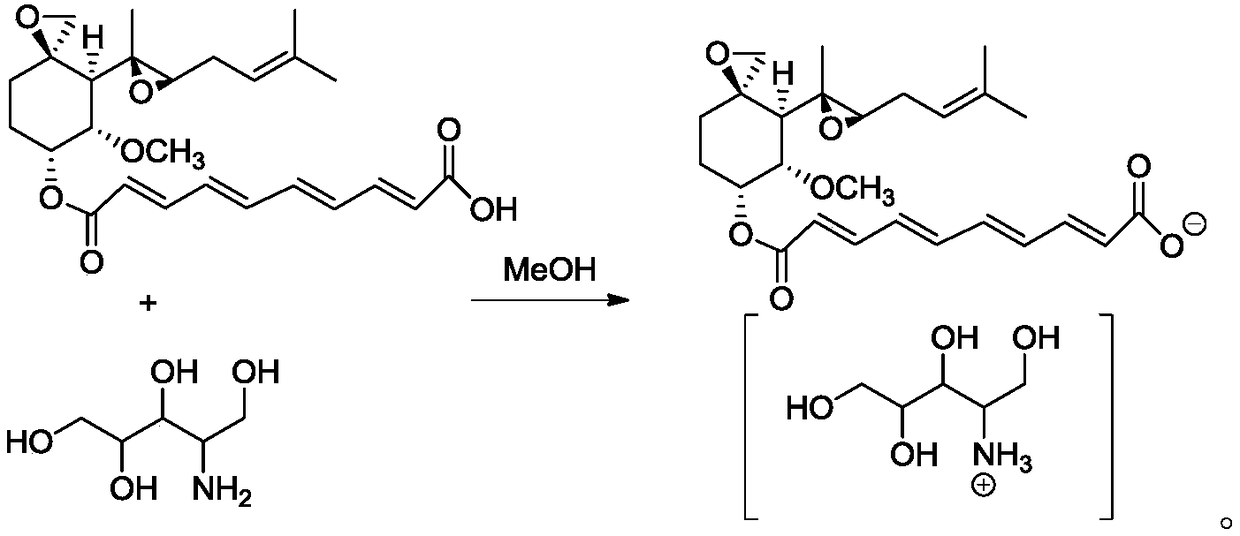
[0032] This embodiment provides a method for synthesizing amino polyols, and the synthetic route is as follows:
[0033]
[0034] The specific operation steps are as follows:
[0035] 1.0g KBH 4 Dissolve it in 17ml of sodium hydroxide (0.05M) solution and gradually add it dropwise to a round bottom flask containing 1.0g of glucosamine hydrochloride. During the addition process, a large number of bubbles will appear (if the temperature rises sharply, vigorous stirring is required) , and slowly added). After reacting at room temperature for 2.5 h, the progress of the reaction was detected by TLC. If the reaction was complete, acetic acid was added to quench the reaction.
[0036] After the reaction solution was repeatedly washed with methanol, it was concentrated by distillation to obtain a white solid with a yield of more than 95% and a purity of 98%.
Embodiment 3
[0038] This embodiment also provides a method for synthesizing amine-based polyols. Compared with Example 2, the only difference is that individual operating parameters are different, specifically: adding NaBH 4 , LiAlH 4 or Pd / H 2 Reducing agent to replace KBH 4 .
[0039] The yield of the product obtained in this example is 80%-90%, and the purity is above 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com