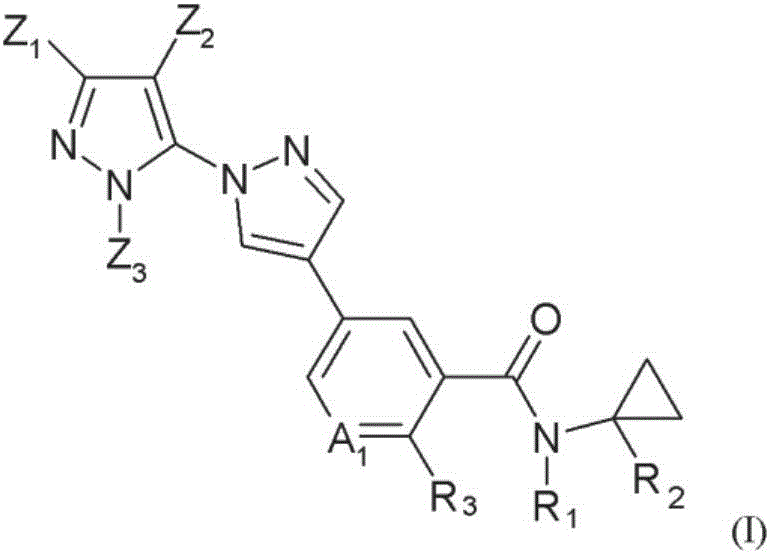
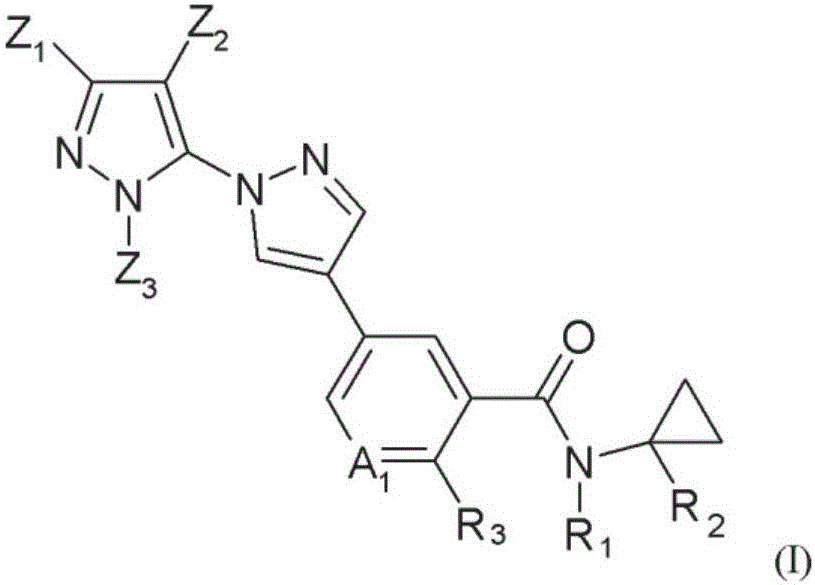
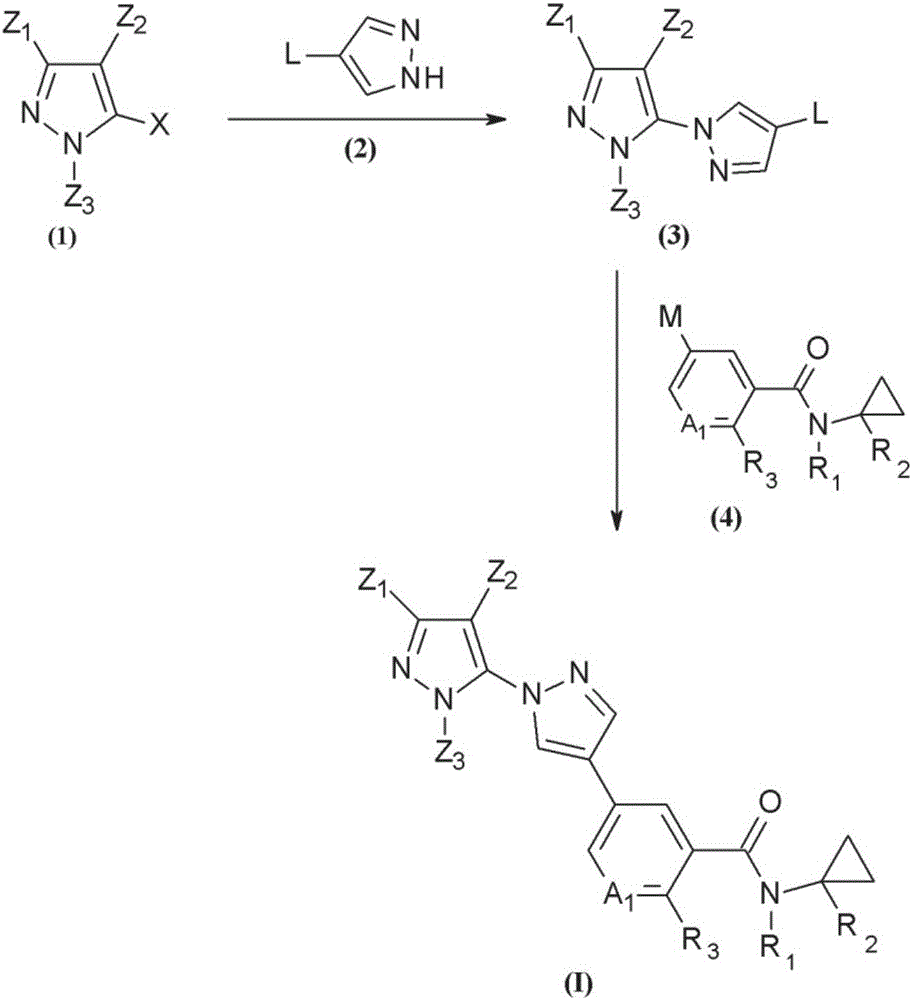
Novel halogen-substituted compounds
A compound, a representative technology, applied in the field of new halogen-substituted compounds, which can solve problems such as resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[2319] Preparation of starting materials
[2320] Synthesis of 3-(1-fluorocyclopropyl)-1-methyl-1H-pyrazole (10)
[2321]
[2322] (2E)-3-(Dimethylamino)-1-(1-fluorocyclopropyl)prop-2-en-1-one (16)
[2323] According to the description of (2E)-3-(dimethylamino)-1-(cyclopropyl)prop-2-en-1-one in J.Med.Chem.2011,54,7974, by (15) Initial synthesis of (2E)-3-(dimethylamino)-1-(1-fluorocyclopropyl)prop-2-en-1-one (16).
[2324] HPLC-MS *) : logP=1.16, mass (m / z)=158[M+H] + .
[2325] 1 H-NMR (400MHz, DMSO-d6): 7.63(d, 1H), 5.47-5.43(m, 1H), 3.12(s, 3H), 2.84(s, 3H), 1.28-1.10(m, 4H)
[2326] 3-(1-fluorocyclopropyl)-1-methyl-1H-pyrazole (10)
[2327] Dissolve 685 mg (4.35 mmol) of (2E)-3-(dimethylamino)-1-(1-fluorocyclopropyl)prop-2-en-1-one (16) in 10 mL of EtOH and add 0.35 mL Methylhydrazine (6.53 mmol). The mixture was stirred overnight at 60 °C. 1.16 mL methylhydrazine (2.18 mmol) was added and stirring was continued for a further 5 hours at 60°C. The mixtur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com