Combination therapy for treatment of cancer
A technology of cancer, therapeutic agent, applied in the field of combination therapy for the treatment of cancer, capable of solving problems such as undifferentiated, uncontrolled proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
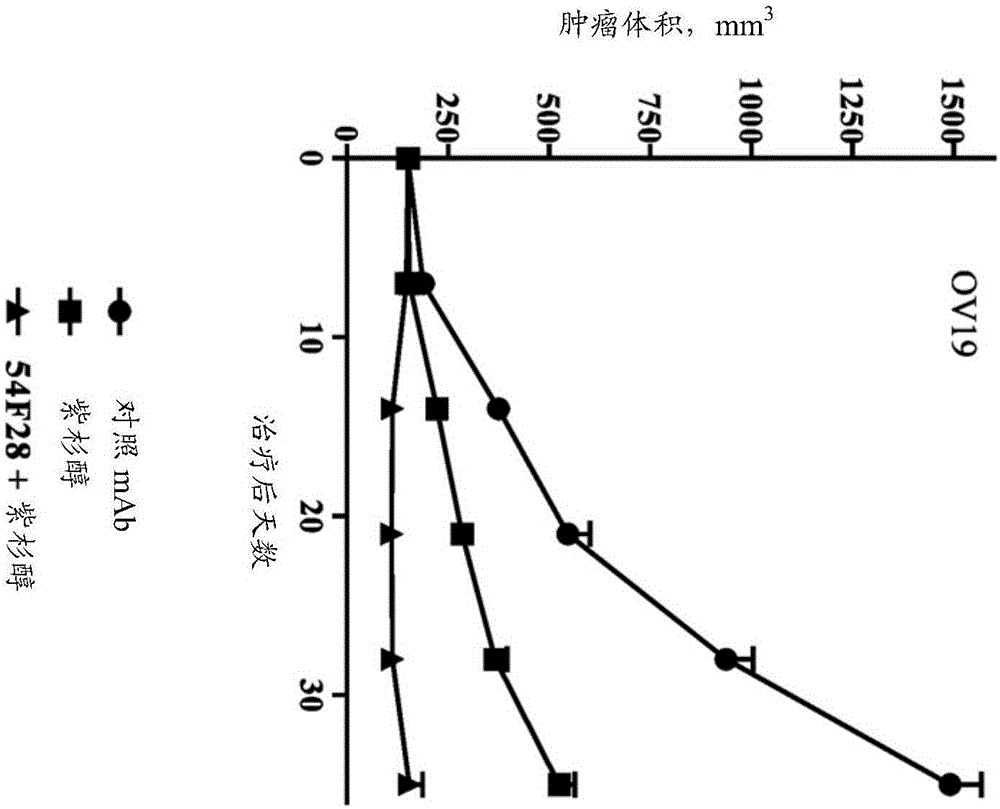
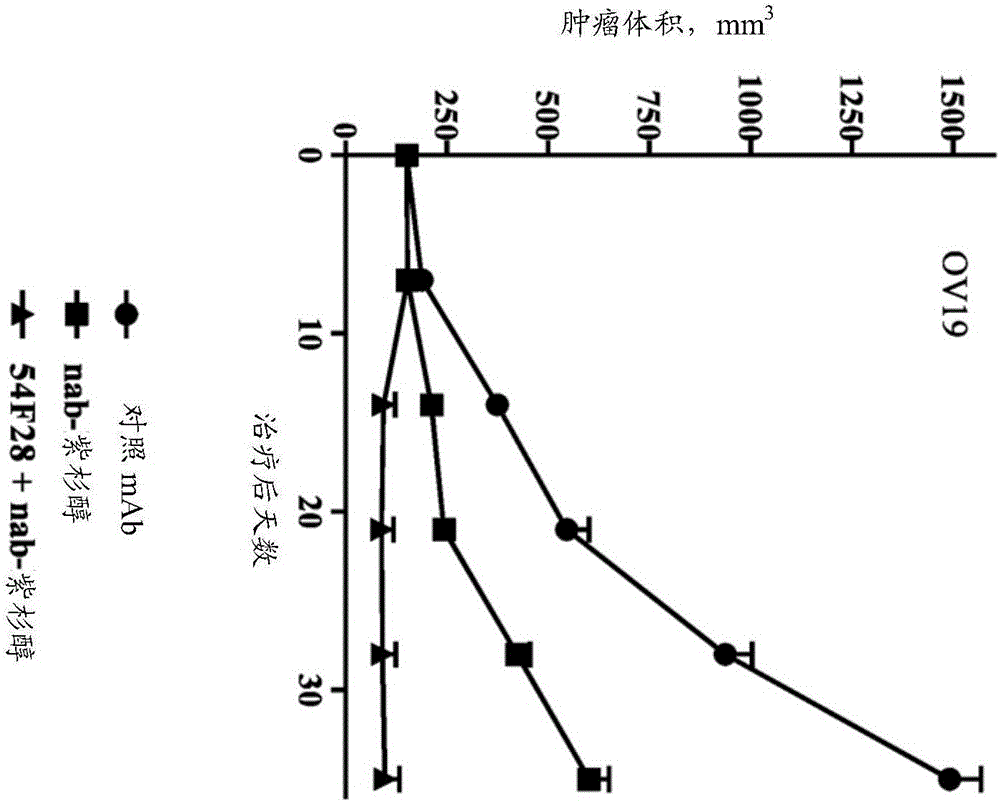
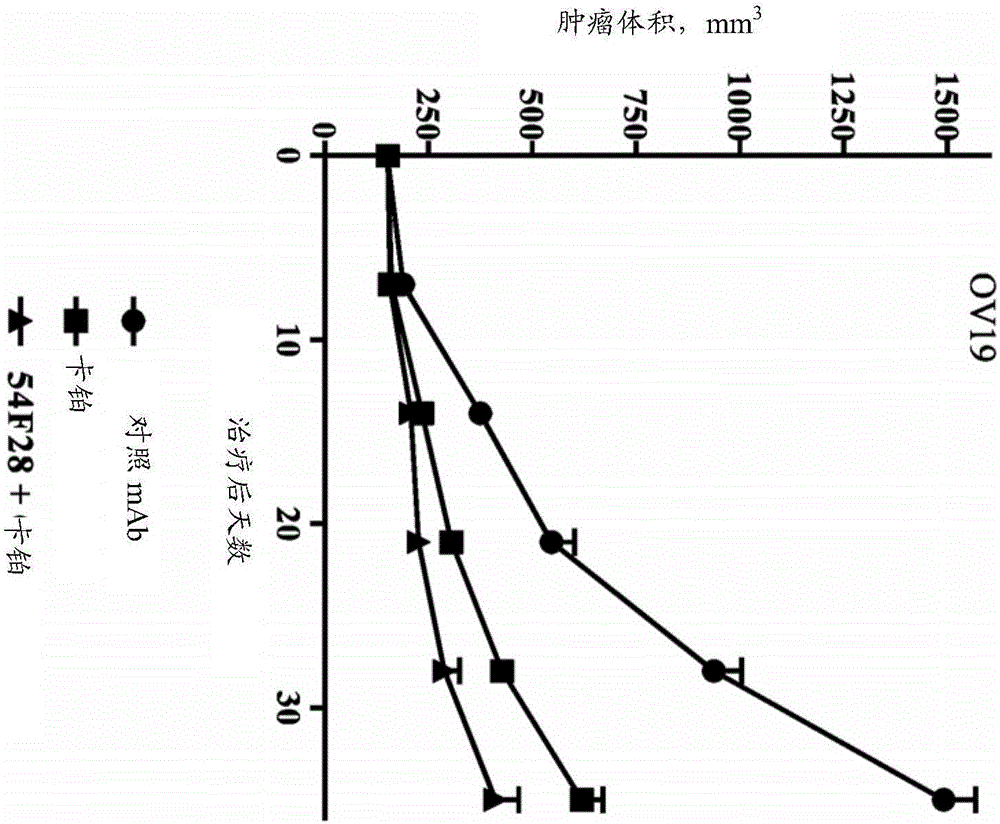
[0298] Activity of FZD8-Fc Soluble Receptor OMP-54F28 in Combination with Chemotherapeutic Agents in Vivo
[0299] The OncoMed xenograft models described herein were established at OncoMed Pharmaceuticals from minimally passaged patient-derived tumor specimens. Tumor samples are examined by a pathologist and classified into specific tumor types. OncoMed relies on these classifications unless further analysis is performed on any particular tumor and reclassification is deemed warranted.
[0300] Xenograft OMP-OV19 ovarian tumor cells (1×10 5 cells) were injected subcutaneously into 6-8 week-old NOD / SCID mice. Tumors were grown for 28 days until they reached 120mm 3 average volume. Mice were randomly divided into groups (n=9 per group) and treated with paclitaxel, nab-paclitaxel, carboplatin, combination of carboplatin and paclitaxel, combination of OMP-54F28 and paclitaxel, combination of OMP-54F28 and nab-paclitaxel Mice were treated with the combination, the combination ...
Embodiment 2
[0305] Effect of Staggered Dosing Regimen on the Activity of Anti-FZD Antibody OMP-18R5 in Combination with Paclitaxel
[0306] Single cell suspensions of xenografted UM-PE13 mammary tumor cells (20,000 cells) were injected subcutaneously into 6-8 week old NOD / SCID mice. UM-PE13 is a type of triple negative breast cancer. Tumors were allowed to grow for 34 days until they reached an average of 80mm 3 volume of. Mice were randomly divided into groups (n=8 per group) and treated with paclitaxel, a combination of OMP-18R5 and paclitaxel administered on the same day, a combination of OMP-18R5 and paclitaxel (where paclitaxel was administered 3 days before the administration of OMP-18R5), A combination of OMP-18R5 and paclitaxel (wherein OMP-18R5 was administered 3 days prior to paclitaxel administration), or a control antibody treated mice. Mice were treated every three weeks with OMP-18R5 at a dose of 25 mg / kg and paclitaxel at a dose of 20 mg / kg. OMP-18R5 and paclitaxel were...
Embodiment 3
[0310] Effect of Staggered Dosing Regimen on the Activity of FZD8-Fc Soluble Receptor OMP-54F28 in Combination with Paclitaxel
[0311] Xenograft OMP-OV38 ovarian tumor cells (1×10 5 cells) were injected subcutaneously into 6-8 week-old NOD / SCID mice. Tumors were grown for 38 days until they reached 140mm 3 average volume. Mice were randomly divided into groups (n=9 per group) and treated with paclitaxel, a combination of OMP-54F28 and paclitaxel administered on the same day, a combination of OMP-54F28 and paclitaxel (where paclitaxel was administered 2 days before the administration of OMP-54F28), OMP Mice were treated with a combination of 54F28 and paclitaxel (where OMP-54F28 was administered 2 days prior to paclitaxel administration) or a control antibody. Mice were treated biweekly with OMP-54F28 at a dose of 25 mg / kg and paclitaxel at a dose of 20 mg / kg. OMP-54F28 and paclitaxel were administered intraperitoneally. Tumor growth was monitored and tumor volume was mea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com