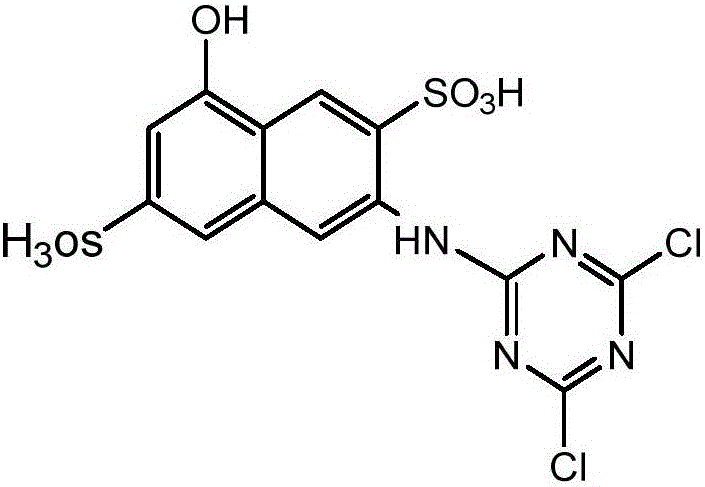
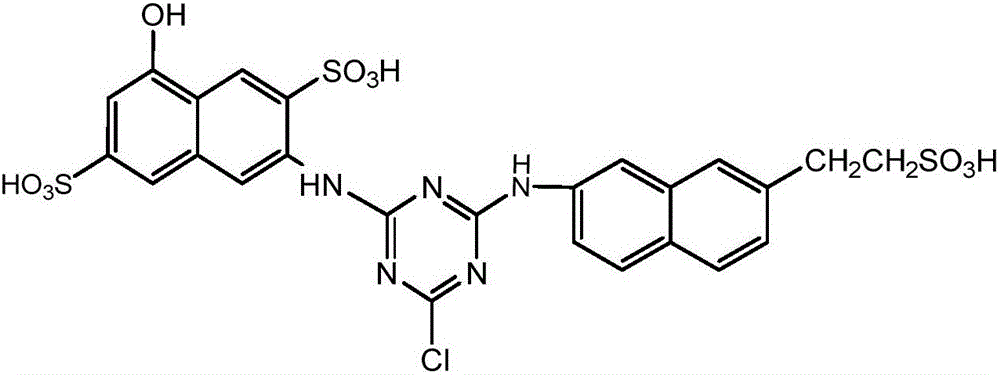
Synthesis method of active orange dye
A synthetic method and technology of orange dye, applied in the direction of reactive dyes, azo dyes, organic dyes, etc., can solve the problems of low solubility of orange dye, impossibility of dyeing by exhaust dyeing, dark color of orange dye, etc., and achieve bright color, high solubility, The effect of simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. One shrinkage reaction
[0032] 1.1 Cyanuric chloride beating
[0033] Add 150 g of ice to a clean 500 mL beaker, add 20 g of cyanuric chloride, 0.5 g of dispersant MF, and grind with ice for 40 minutes.
[0034] 1.2 Para-ester dissolution
[0035] Add 31g of para-ester to a clean 500mL beaker, add 200mL of water, keep the temperature around 15°C, stir and beat, and adjust the pH of the solution to 5.5-6.0 with baking soda while stirring. until the para-ester is completely dissolved.
[0036] 1.3 A contraction reaction
[0037] Add the dissolved para-ester solution dropwise to the cyanuric chloride solution, and add it in about 15 minutes. During the addition process, the temperature should be strictly controlled at 0-6 °C, and the temperature of the condensation process should be controlled at 0-5 °C. After adding the solution, slowly adjust the pH to 3.0-3.5 with baking soda, continue to maintain pH=3.0-3.5, and stir the reaction at a temperature of 0-5 °C unti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com