A kind of synthetic method of pidotimod
A synthetic method, the technology of pidotimod, which is applied in the field of pidotimod synthesis, can solve the problems of reducing racemization, no racemization, etc., and achieve the effects of solving the racemization problem, increasing the yield, and shortening the synthesis cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
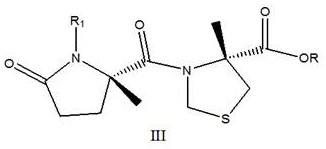
[0030] Example 1, a Synthesis of President: This method employs a carboxy R group-protected L-thiazolidine-4-carboxylic acid ester or L-thiazolin-4-carboxylic acid and nitrogen R1 protection Glutamic acid is a catalyst in which R2 substituted with boric acid, the condensation forms the target compound shown by the formula III:
[0031]
[0032] The R is selected from: H, OTBU, BN, CHX, MPE, 2-pH1Pr, TEGBN, DAMB, Al, PNB, PTMSE or DMNB;
[0033] The R1 is selected from: , IVDDE, FMOC *, MTT or Alloc;
[0034] The R2 is selected from the group consisting of each benzene ring, a substituted benzene ring, furan, thiophene, imidazole or diazole.
Embodiment 2
[0035] Example 2, a Synthesis of a President described in Example 1: The substituted benzene ring is selected from the group consisting of 3-bit fluoride substitution, 3,5-spin substitution, 3 chlorine substitution or trifluoromethyl substitution Benzene ring.
[0036] The specific steps are as follows:
[0037] (1) A nitrogen R1-protected cokeline, R2 substituted boric acid, activated molecular sieve, organic solvent together, uniform uniform; activated molecular sieve with a molecular sieve of a diameter of 4 å; the organic solvent is anhydrous chloride Ethyl methane or ethyl acetate;
[0038] (2) Add a carboxyl R group-protected L-thiazolidine-4-carboxylic acid ester or L-thiazolidine-4-carboxylic acid, continuing the stirring reaction;
[0039] (3) After the reaction is completed, the reaction liquid is filtered by filtrate;
[0040](4) When the raw material uses a carboxyl R group-protected L-thiazolidine-4-carboxylate, nitrogen-free protective pangular amine, the filtrate is...
Embodiment 3
[0048] Embodiment 3, in the synthesis method of a President described in Example 1:
[0049] Raw nitrogen R1 protected cokeline, carboxy R group protected L-thiazolin-4-carboxylic acid ester or L-thiazolidine-4-carboxylic acid, R2 substituted boric acid molar ratio is 1.5: 1: 0.3 ;
[0050] Organic Solvent Volume ML and Nitrogen R1ONBS, DNBS, Troc, DTS, PNZ, ONZ, NVOC, NPPOC, HFA, DDZ, BPOC, NPS, NSC, BSMOC, α-NSMOC, IVDE, FMOC *, MTT or Alloc;
[0051] The R2 is selected from the group consisting of each benzene ring, a substituted benzene ring, furan, thiophene, imidazole or diazole.
[0052] Example 2, a Synthesis of a President described in Example 1: The substituted benzene ring is selected from the group consisting of 3-bit fluoride substitution, 3,5-spin substitution, 3 chlorine substitution or trifluoromethyl substitution Benzene ring.
[0053] The specific steps are as follows:
[0054] (1) A nitrogen R1-protected cokeline, R2 substituted boric acid, activated molecular s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com