Method for preparing efinaconazole intermediate
A technology for chiral ligands and compounds is applied in the field of preparation of efluconazole intermediates, and can solve the problems of high cost, large amount of reaction reagents, cumbersome reaction steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
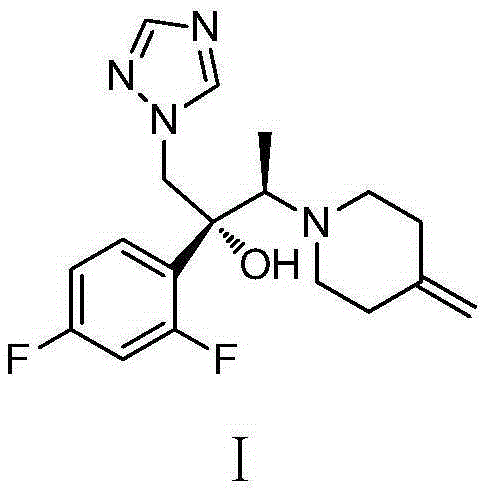
[0114] Example 1: (2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylenepiperidin-1-yl)-1-(1H-1,2,4- Preparation of Triazol-1-yl)butan-2-ol (Efluconazole)
[0115]
[0116] Step 1: Preparation of (2R)-2-(2,4-difluorophenyl)-1-(1H-1,2,4-triazol-1-yl)butan-2,3-diol.
[0117] To a 500 mL reaction flask was added water (94 mL), potassium ferricyanide (39.5 g, 120 mmol), potassium carbonate (16.6 g, 120 mmol), methanesulfonamide (3.80 g, 40 mmol) , potassium osmate dihydrate (141.5 mg, 0.4 mmol), hydroquinidine 1,4-(2,3-naphthyridine) diether (1.56 g, 2.0 mmol), tert-butanol (94 mL ) and 1-(2-(2,4-difluorophenyl)but-2-enyl)-1H-1,2,4-triazole (9.41 g, 40 mmol). The whole mixture was stirred at room temperature for 40 hours.
[0118] Ethyl acetate (40 mL) and 30% aqueous sodium sulfite (55 mL) were added to the reaction system. The organic phase was separated for use; the aqueous phase was extracted with ethyl acetate (40 mL), and the organic phase was separated; the organic phases wer...
Embodiment 2
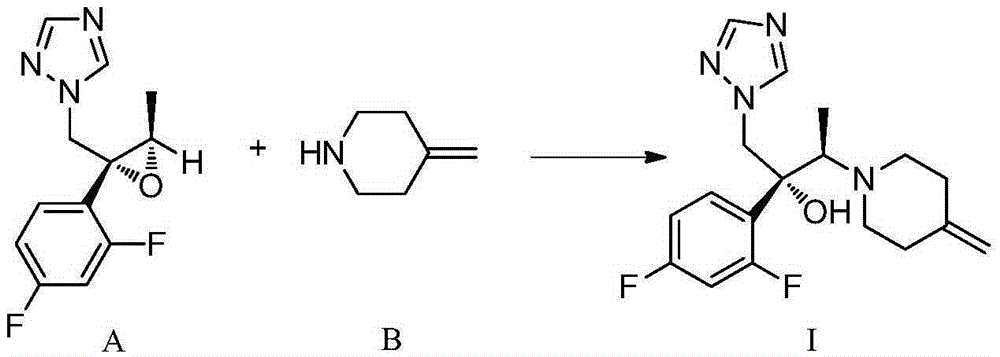
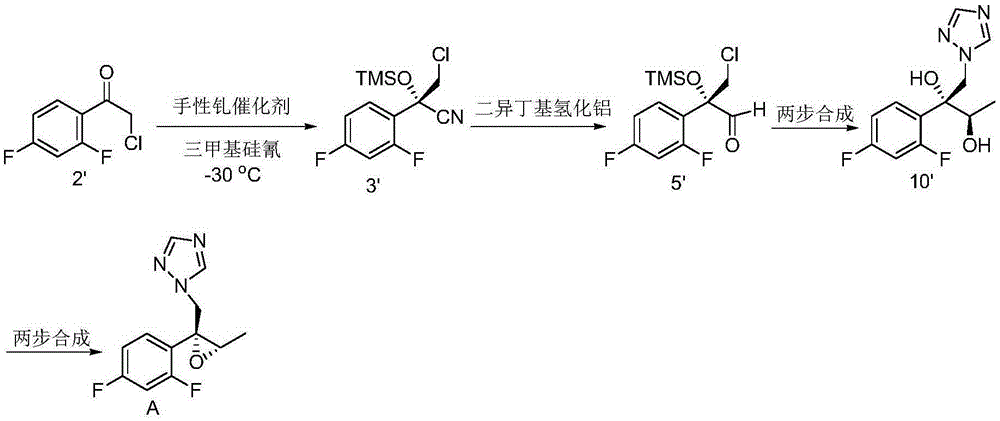
[0128] Example 2: 1-(((2R,3S)-2-(2,4-difluorophenyl)-3-methylepoxy-2-yl)methyl)-1H-1,2,4- Preparation of triazole (intermediate A)
[0129]
[0130] Step 1: Preparation of (R)-2-(2,4-difluorophenyl)-3-(1H-1,2,4-triazol-1-yl)propane-1,2-diol
[0131] To a 500 mL reaction flask was added water (66 mL), potassium ferricyanide (29.6 g, 90 mmol), potassium carbonate (12.4 g, 90 mmol), methanesulfonamide (2.85 g, 30 mmol) , potassium osmate dihydrate (106 mg, 0.3 mmol), hydroquinidine 1,4-(2,3-naphthyridine) diether (1.17 g, 1.5 mmol), tert-butanol (66 ml ) and 1-(2-(2,4-difluorophenyl)allyl)-1H-1,2,4-triazole (6.63 g, 30 mmol). The whole mixture was stirred at room temperature for 24 hours.
[0132] Ethyl acetate (30 mL) and 30% aqueous sodium sulfite (45 mL) were added to the reaction system. The organic phase was separated for use; the aqueous phase was extracted with ethyl acetate (30 mL), and the organic phase was separated; the organic phases were combined, washed with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com