Application and separation and purification method of monomer compound in wisteria tumor
A technology for separation and purification of compounds, applied in the field of natural botanical pesticides, to achieve the effects of good antibacterial activity, good drug safety and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 1. Take 15kg of dried wisteria medicinal material, pulverize it, and use 80vt% ethanol as a solvent to reflux extract three times at 80°C for 2 hours each time, combine the extracts, recover the extraction solvent, and concentrate to obtain 2kg of extract with a relative density of 1.1 ;
[0047] 2. Gradient elution of the obtained extract with a silica gel column. The eluent is composed of dichloromethane and methanol. The volume ratio of dichloromethane and methanol in the eluent is 100:0~0:1. Gradient elution separation from small to large, TLC detection, 8 components were obtained after merging the same components, which were called Z in order of polarity from small to large 1 -Z 8 ;
[0048] 3. Component Z 4 (82.3g) was eluted through gradient silica gel column, the eluent was composed of petroleum ether and ethyl acetate, the volume ratio of petroleum ether and ethyl acetate in the eluent was 50:1~0:1, the polarity of the eluent Separation by gradient elution ...
Embodiment 2
[0059] 2.1 Instruments and reagents
[0060] 2.1.1 Instruments
[0061] Shimadzu LC-15C high performance liquid chromatography (SPD-15C ultraviolet detector, LC-15C pump, LC-Solution chromatographic workstation, Tianjin Dongkang DT-230A column thermostat), KQ2200 ultrasonic cleaner (Jiangsu Kunshan Ultrasonic Instrument Co., Ltd.), UV759CRT ultraviolet-visible spectrophotometer (Shanghai Youke Instrument Co., Ltd.), German Sartorius CP225D 1 / 100,000 electronic balance.
[0062] 2.1.2 Drug testing
[0063] Acetonitrile and methanol were chromatographically pure (Tianjin Damao Chemical Reagent Factory), water was Wahaha purified water (Hangzhou Wahaha Group Co., Ltd.), and phosphoric acid was analytically pure (Shanghai Suyi Chemical Reagent Co., Ltd.).
[0064] 2.2 Chromatographic analysis conditions
[0065] The experimental chromatographic column is Phenomenex-C 18 (250mm×4.6mm, 5μm), mobile phase is acetonitrile (A)-0.1% phosphoric acid aqueous solution (B), elution cond...
Embodiment 3
[0072] 3.1 Preparation of test sample solution
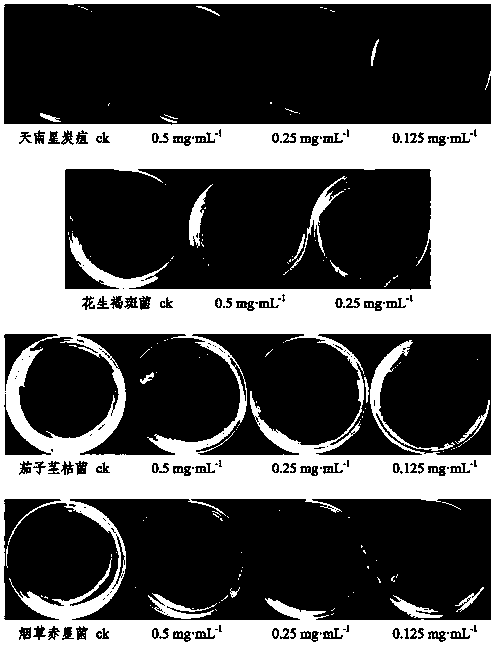
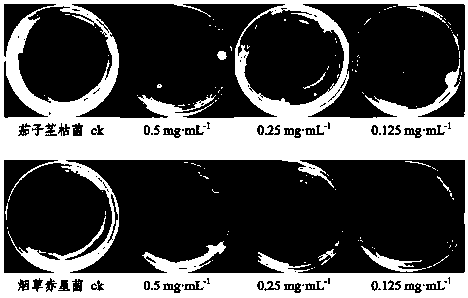
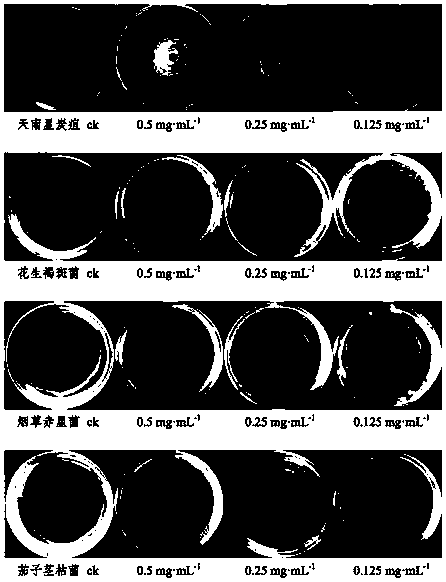
[0073] Select the isolated compounds 3ˊ,5ˊ-dihydroxy-2ˊ,4ˊ,7-trimethoxyisoflavane, 2ˊ,4ˊ,4-trihydroxychalcone, 12-oleanene-3,22,24- Dissolve an appropriate amount of triol in sterile water (if necessary, add a small amount of DMSO to aid in dissolving), and prepare 0.5 mg·ml respectively -1 , 0.25mg·ml -1 , 0.125mg·ml -1 concentrated drug stock solution.
[0074] 3.2 Preparation of drug-containing medium
[0075] Measure 1mL of each concentration of the test drug solution, filter it through a 0.22μm filter membrane, mix it with 9mL of sterilized PDA medium (potato dextrose agar medium) in a sterile test tube, and pour it into a sterilized petri dish Make a culture medium plate containing medicine, and use sterile water and DMSO as blank control.
[0076] 3.3 Preparation of test pathogenic fungus cake
[0077] Inoculate the four crop pathogenic fungi of Aracea anthracnose, peanut brown spot, eggplant stem blight, and tobacc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com