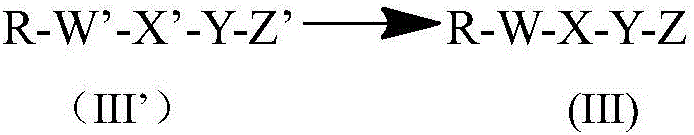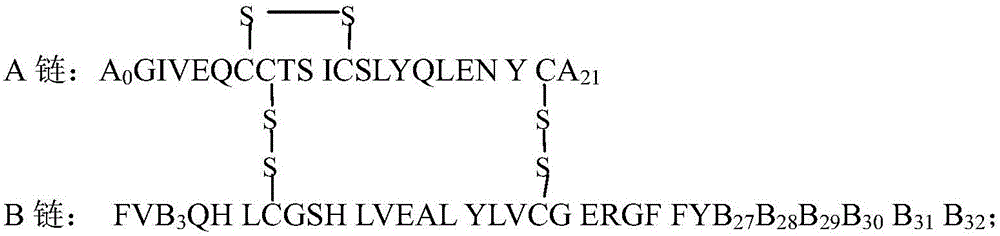Preparation method of acylation derivative of human insulin or analog thereof
A technology for human insulin and analogs, applied in the field of preparation of acylated derivatives, can solve the problems of uneconomical preparation of final products, difficulty in purification, decreased yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1: B28D-N εB29 -(N α -(HOOC(CH 2 ) 14 Preparation of CO)-γ-Glu)-B30E Human Insulin
[0081] 1. Preparation of methylhexadecanedioyl-Glu(OSu)-OMe
[0082] synthetic route:
[0083]
[0084]Take X01 (150g, 524.5mmol), add dry THF (2.5L) at room temperature, then add a catalytic amount of DMF (1.0mL), add oxalyl chloride (49mL) to a 100mL constant pressure dropping funnel, The acid chloride is slowly added dropwise into the reaction flask, during which gas is generated, and the gas needs to be released continuously, and the dropwise addition takes about two hours. After the dropwise addition, stir at room temperature for 1.5 h, then spin dry THF under reduced pressure, add DCM (800 mL) and tert-butanol (500 mL) into the reaction flask, and stir overnight at room temperature. Spin dry tert-butanol and dichloromethane, add dichloromethane (1 L), filter to remove insoluble solids. The filtrate was spin-dried and passed through the column. The required compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com