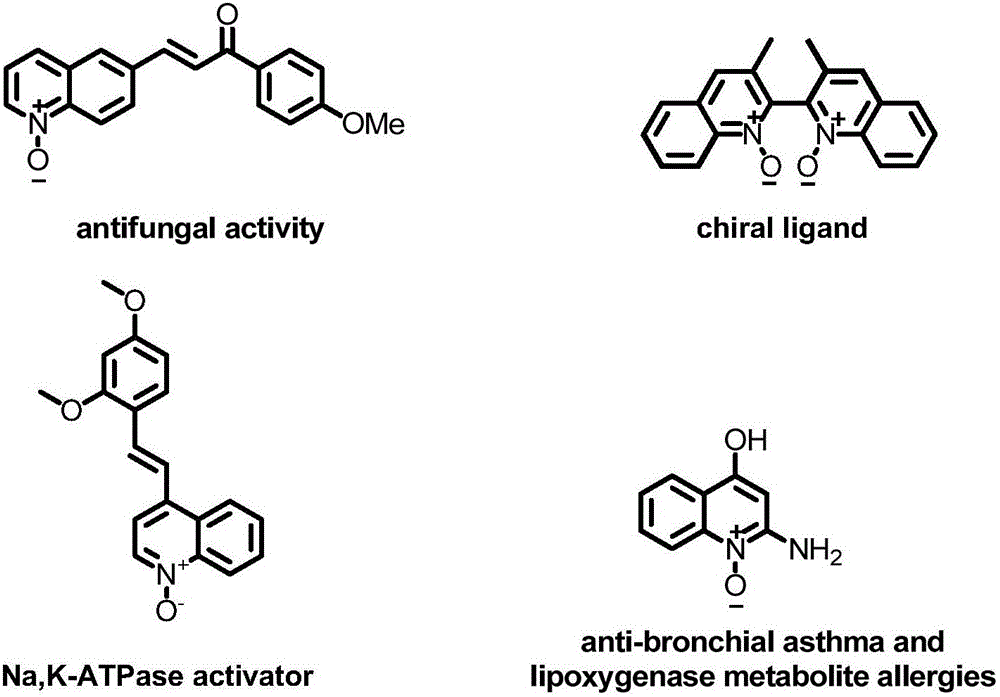
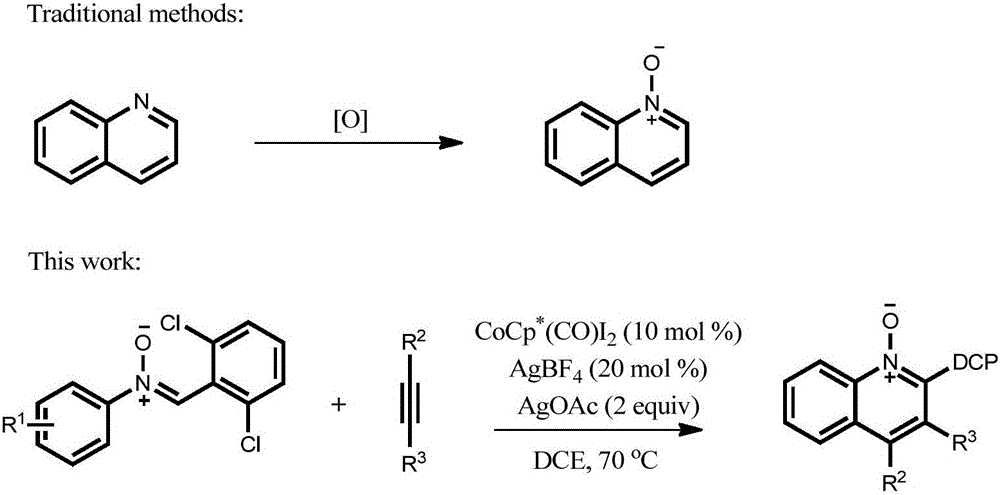
Preparation method of 2,3,4-trisubstituted quinoline-N-oxide compound
A technology for quinoline nitroxides and compounds, which is applied in the field of preparation of quinoline nitroxides and achieves the effect of wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The present invention will be further described below in conjunction with specific embodiments.
[0031] Add nitrone (II) (0.3mmol), alkyne (III) (0.2mmol), catalyst Cp * Co(CO)I 2 (0.02mmol), silver tetrafluoroborate (0.04mmol), silver acetate (0.4mmol) and 2ml of organic solvent, mixed and stirred evenly, after completing the reaction according to the reaction conditions in Table 2, washed, dried, mixed with silica gel, passed through the column layer Analyze and purify to obtain corresponding quinoline nitrogen oxide compound (I), the reaction process is shown in the following formula:
[0032]
[0033] Table 1
[0034]
[0035] Table 2
[0036]
[0037] In Table 1 and Table 2, T is the reaction temperature, t is the reaction time, Me is methyl, DCP is 2,6-dichlorophenyl, Cp * is pentamethylcyclopentadienyl, Ph is phenyl.
[0038] In the present invention, the preparation method of raw materials can refer to: Wang, C.X.; Wang, D.; Yan, H.; Wang, H.; Pan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com