Post-treatment method for 4,6-dihydroxypyrimidine synthesis
A technology of dihydroxypyrimidine and negative pressure, applied in the field of organic chemical industry, can solve the problems of high production cost and large amount of waste acid.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
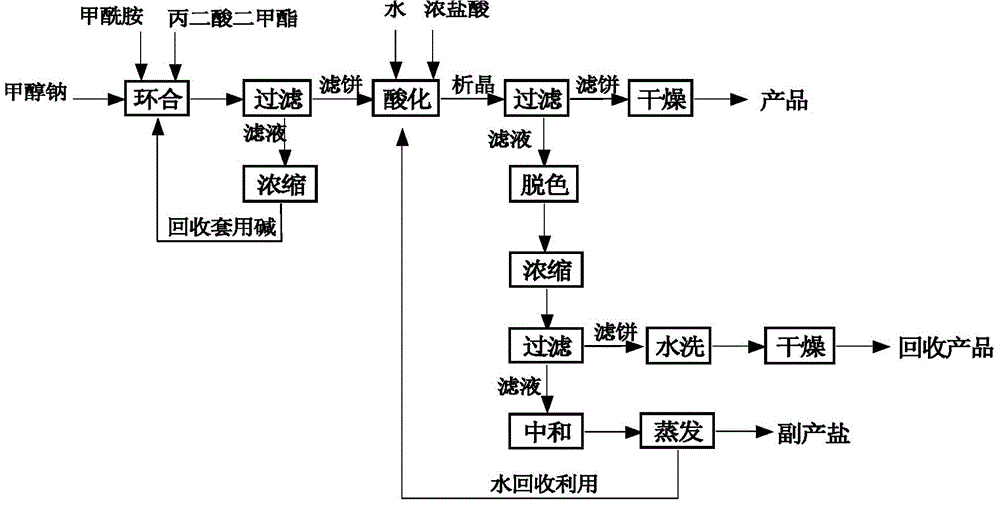
[0025] Synthesis: Add 720g (4mol) of 30% methanol solution of sodium methoxide and 112.5g (2.5mol) of formamide to a four-necked reaction flask, stir, heat up in a water bath, heat up to 50°C, and start to dropwise add 132g (1mol) of propanediol For dimethyl ester, the temperature is controlled at 50-62°C during the dropwise addition process, and it takes about 1 hour. After the dropwise addition, the temperature is raised to reflux temperature of 62-66°C, kept for 2 hours, and then lowered to room temperature.
[0026] Post-processing: the above reaction solution is subjected to negative pressure suction filtration to obtain filtrate 580g (analytical content: sodium methylate: 9.31%; formamide: 0.59%), and the concentration of negative pressure concentration to sodium methylate is about 35%. After reclaiming methanol , and the filtrate is used for recovery of alkali. At the same time, add 400g of water to dissolve the filter cake, keep the temperature of the kettle not exceed...
Embodiment 2
[0030] Synthetic process is the same as example 1.
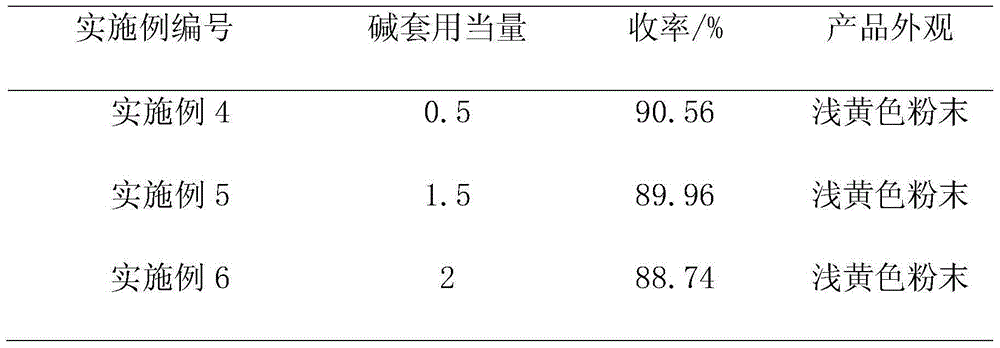
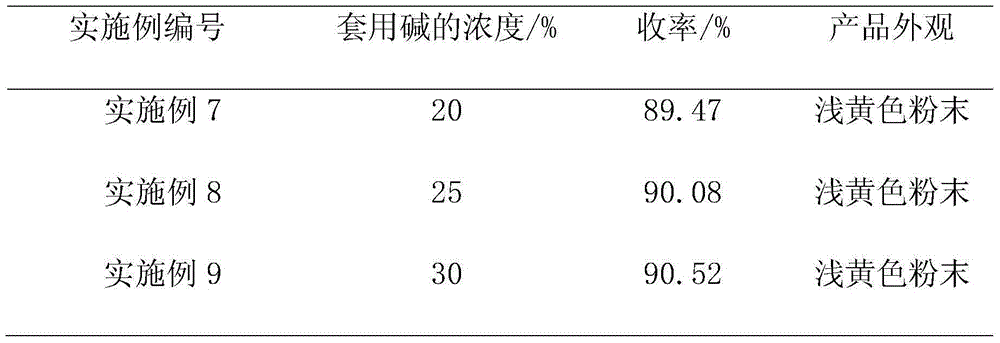
[0031] Post-processing: the above-mentioned reaction solution is subjected to negative pressure suction filtration to obtain 600 g of filtrate (analytical content: sodium methylate: 7.86%; formamide: 0.43%), and the concentration of negative pressure concentration to sodium methylate is about 30%. After reclaiming methanol , and the filtrate is used for recovery of alkali. At the same time, add 350g of water to dissolve the filter cake, keep the temperature of the kettle not exceeding 30°C, adjust the pH to 2-3 to crystallize with 240g of industrial hydrochloric acid, keep stirring at room temperature for 1 hour, cool down to below 10°C, and filter under negative pressure. Under vacuum condition -0.09±0.01MPa, dry to obtain 4,6-dihydroxypyrimidine. The base is applied mechanically at 1.5 equivalents, that is, the recovery rate is 37.5%, and applied mechanically twice, an average of 96.9g of 4,6-dihydroxypyrimidine is obtain...
Embodiment 3
[0035] Synthetic process is the same as example 1.
[0036]Post-processing: the above-mentioned reaction solution is subjected to negative pressure suction filtration to obtain filtrate 568g (analysis content: sodium methylate: 9.51%; formamide: 0.38%), and the concentration of negative pressure concentration to sodium methylate is about 30%. After reclaiming methanol , and the filtrate is used for recovery of alkali. At the same time, add 380g of water to dissolve the filter cake, keep the temperature of the kettle not exceeding 30°C, adjust the pH to 2-3 to crystallize with 250g of industrial hydrochloric acid, keep stirring at room temperature for 1 hour, cool down to below 10°C, and filter under negative pressure. Under vacuum condition -0.09±0.01MPa, dry to obtain 4,6-dihydroxypyrimidine. The base is applied mechanically at 2 equivalents, that is, the recovery rate is calculated as 50%, and applied mechanically twice in a row to obtain an average of 95.6g of 4,6-dihydrox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com