Cefuroxime sodium powder preparation for injection
A technology of cefuroxime sodium and fururoxime sodium powder, which is applied in the direction of medical preparations containing active ingredients, powder delivery, antibacterial drugs, etc., and can solve the problems of poor stability and color of cefuroxime sodium that affect the quality and safety of drugs. Rapid changes and other issues, to achieve the effect of improving the national health level, significant social benefits, and good color of the finished product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] Step A, preparation of aseptic sodium agent solution: stir and dissolve anhydrous sodium acetate in a reaction tank with methanol, then add an adsorbent to stir and decolorize, filter, and aseptically treat to obtain a sterile sodium agent solution for subsequent use;
[0024] Step B. Preparation of sterile cefuroxime acid solution: add acetone and water into a dry and clean dissolving tank, stir and add cefuroxime acid to dissolve under nitrogen protection conditions, then add an adsorbent and stir to decolorize, filter, and aseptically treat to obtain Sterile cefuroxime acid solution, standby;
[0025] Step C, crystallization: add a small amount of acetone to the crystallization tank, control the temperature at 8-10°C, and add the sterile sodium-forming agent solution prepared in step A and the sterile cefuroxime prepared in step B to the crystallization tank at the same time for 2-4 hours Caprylic acid solution and acetone, and then grow the crystal for 0.8-1.5h once...
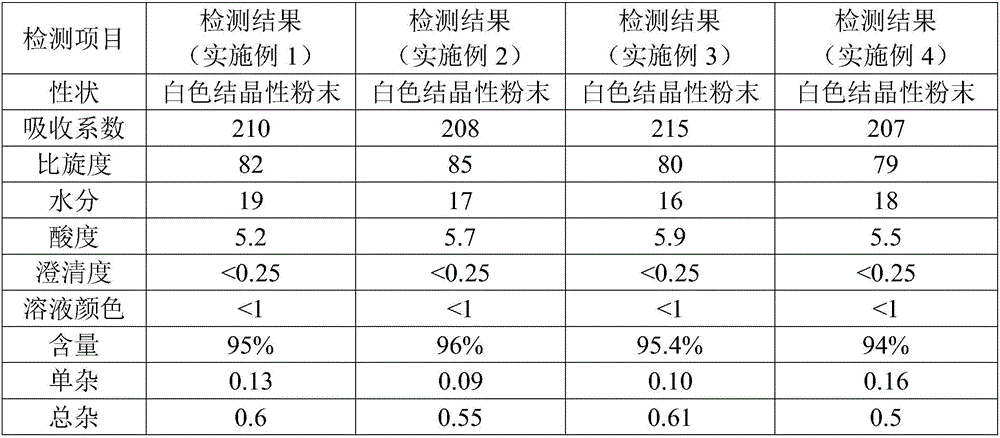
Embodiment 1
[0036] Step A, preparation of aseptic sodium agent solution: Stir and dissolve 18Kg anhydrous sodium acetate in a stainless steel reaction drying tank with 200L methanol, then add 5Kg adsorbent and stir at 10°C for 30min, filter, and aseptically treat to obtain aseptic product Sodium solution, spare;
[0037] Step B. Preparation of sterile cefuroxime acid solution: add 750L of acetone and 60L of purified water into a dry and clean dissolving tank, stir and add 85Kg of cefuroxime acid until dissolved under nitrogen protection, then add 5Kg of adsorbent and stir at 10°C for 30min to decolorize , filtered, aseptically processed, and pressed into a sterile cefuroxime acid solution tank to obtain a sterile cefuroxime acid solution for subsequent use;
[0038] The adsorbent in step A and step B is activated carbon or wood fiber or a mixture of the two, the stirring speed of the adsorbent is 60r / min, the filter is decarburized by 3-8μm titanium rod, and the aseptic treatment is to pa...
Embodiment 2
[0042] The difference between this embodiment and embodiment 1 is:
[0043] The stirring speed of the adsorbent in step A and step B is 40r / min, and a 6-8μm titanium rod is used for filtration and decarburization.
[0044] Step C, crystallization: After adding 50L of acetone to the crystallization tank, control the temperature of the crystallization tank at 9°C, and add the sterile sodium forming agent solution in step A and the sterile cefuroxime acid solution in step B to the crystallization tank at the same time for 4 hours and 1000L acetone for 1.3 hours of crystal growth; then continue to add 1500L of acetone in the form of gradient drop for 1.5 hours, and lower the temperature of the crystallization tank to 1°C for the second crystal growth of 1.8h, and then filter and wash the crystal grains Drying makes it loose, and the loose crystal grains are transferred to a vacuum dryer for drying, and after passing the inspection, cefuroxime sodium sterile powder bulk drug for in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com