Preparation method of ALS (Amyotrophic Lateral Sclerosis) patient specific motor neuron
A motor neuron and specific technology, applied in the biological field, can solve problems such as large side effects, covering up key factors, and inability to truly reflect the pathogenic mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Example 1. Obtaining induced pluripotent stem cells (iPSC) derived from ALS patients
[0097] 1. Obtaining induced pluripotent stem cells derived from ALS patients
[0098] 1. Cell culture
[0099] Two lines of skin fibroblasts derived from ALS patients carrying different gene mutations: FUS G1566A fibroblasts (ND29563) and SOD1 A272C fibroblasts (ND29149) were expanded in fibroblast culture medium. After the cells were cultured to a suitable density, they were digested and counted, and 1.5×10 6 indivual.
[0100] 2. Preparation of electrotransfer solution
[0101] Mix 100 μl opti-MEM (product of life technology), 1.5 μg pCXLE-hOCT3 / 4-shp53-F, 1.5 μg pCXLE-hSK, 1.5 μg pCXLE-hUL and 1.5 μg pCXLE-EGFP to obtain electrotransfer solution, and place at 1.5 ml EP tube.
[0102] 3. Use the electrotransfer solution obtained in step 2 to resuspend the FUS G1566A fibroblasts (ND29563) and SOD1 A272C fibroblasts (ND29149) obtained in step 1, respectively, transfer them to the...
Embodiment 2
[0113] Example 2. Targeted correction of gene mutations carried by two ALS-iPSCs
[0114] Utilize CRISPR / Cas9 gene editing technology to two kinds of iPSC (FUS) obtained in embodiment 1 G1566A - iPSC and SOD1 A272C -iPSCs) carry out targeted elimination of gene mutations, and finally obtain iPSCs that eliminate gene mutations. Specific steps are as follows:
[0115] 1. Cell culture
[0116] The two iPSCs (FUS G1566A - iPSC and SOD1 A272C -iPSC), the medium is mTESR. When the clone density reaches 70%-80%, digest with TrypLE, count and collect 5×10 6 cells.
[0117] 2. Preparation of electrotransfer solution
[0118] Mix 8 μg of Cas9-GFP plasmid, 8 μg of the corresponding gene-corrected recombinant template and 4 μg of the corresponding gene-corrected gRNA-mCherry plasmid, and dilute to 100 μl with opti-MEM (life technology) to obtain the electrotransfer solution, which is placed in a 1.5ml EP tube .
[0119] When the relevant gene mutation to be corrected is FUS G156...
Embodiment 3
[0137] Example 3. Obtaining and identification of motor neurons carrying ALS pathogenic gene mutations and gene-corrected motor neurons
[0138] 1. Obtaining motor neurons carrying ALS pathogenic gene mutations and gene-corrected motor neurons
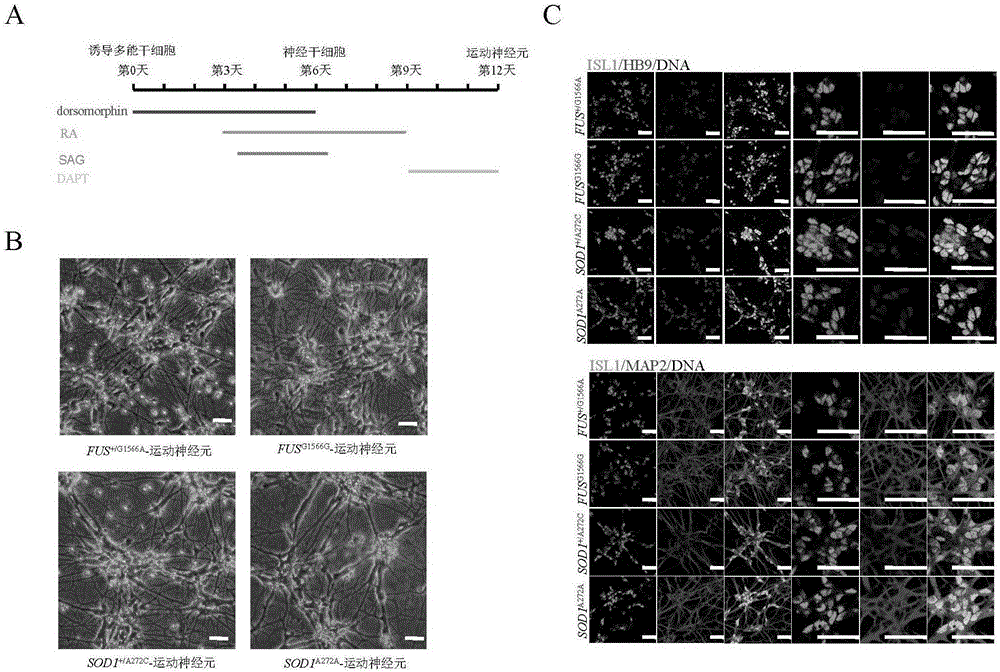
[0139] The two iPSCs (FUS) carrying gene mutations obtained in Example 1 were G1566A - iPSC and SOD1 A272C -iPSC) and two kinds of gene-corrected iPSCs obtained in Example 2 (FUS G1566G - iPSC and SOD1 A272A -iPSCs) were directedly induced to differentiate into motor neurons at the same time, and FUS were obtained respectively G1566A -MN,SOD1 A272C -MN, FUS G1566G -MN,SOD1 A272A -MN. Schematic diagram of the protocol for directed differentiation of iPSCs into motor neurons. image 3 Shown in A. The specific operation is as follows:
[0140] 1. Inoculate the four small iPSC clones obtained in Example 1 and Example 2 into a culture plate previously covered with MEFs treated with mitomycin inactivation, and culture them with cDF...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com