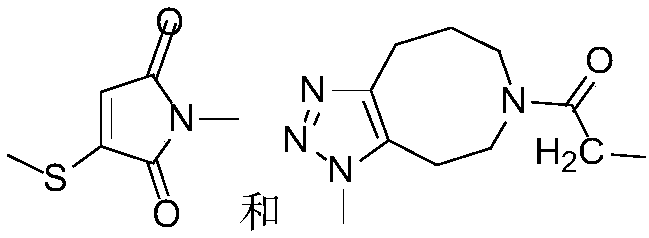
Photocleavage fluorescent labeling compound and application
A Fluorescent Labeling, Compound Technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] A method for synthesizing a photocleavable fluorescently labeled reversible terminal compound, comprising any one of the following routes 1-5:
[0079] Synthetic route 1:
[0080]
[0081] Wherein, the reaction conditions of step (i) are: compound 10mmol 2.5g A.1 in tert-butyldimethylsilyl chloride (TBSCl) 4.82g 32mmol, imidazole (imidazole) 4.5g 66mmol and N,N-dimethylformazol Under the condition that amide (DMF) 30ml exists, room temperature, overnight, then reaction solution is di-tert-butyl methyl dicarbonate ((Boc) 2 O) 20mmol 4.36g, 4-dimethylaminopyridine (DMAP) 10mmol 1.22g and N,N-dimethylformamide (DMF) 30ml under the condition of existence, room temperature, overnight, after vacuum concentration, the residue was saturated with Ammonium chloride solution washed twice (50ml each time), using CH 2 Cl 2 Extract the combined aqueous layer, dry the combined organic layer with sodium sulfate, concentrate in vacuo, and purify by silica gel column chromatography...
Embodiment 2
[0160] A method for synthesizing a photocleavable fluorescently labeled reversible terminal compound, comprising any one of the following routes 1-5:
[0161] Synthetic route 1:
[0162]
[0163] Wherein, the reaction condition of step (i) is: 2.9g 5mmol compound A.3 in 5.1g 15mmol 4-iodo-2-nitrobenzyl bromide (4-iodo-2-nitrobenzyl bromide), 20mgNaH and 50mlN,N-di The reaction was stirred at room temperature for 4 h in the presence of methylformamide (DMF), dried over sodium sulfate, concentrated in vacuo, and purified by silica gel column chromatography to obtain Compound A. 93.5 g (84%);
[0164] In the above step (i), the 4-iodo-2-nitrobenzyl bromide added can be any value in 10-20 mmol;
[0165] The reaction condition of step (ii) is: the compound A.9 obtained in step (i) is in NaN 3 , CuI, L-proline, NaOH and DMSO were reacted for 2 h, concentrated in vacuo, and purified by silica gel column chromatography to obtain 2.9 g (91%) of compound A.10;
[0166] In the abov...
Embodiment 3
[0234] In order to detect whether the reversible terminal synthesized by the present invention can be applied to DNA / RNA sequencing, this example further tested the A.8, G.8, C.8, T.8, U. 8. Two characteristics of the reversible terminal compounds of A.14, G.14, C.14, T.14, U.14:
[0235] 1) Whether it can be recognized by DNA polymerase and participate in DNA extension reaction as a substrate of DNA polymerase;
[0236] 2) Whether the fluorescent group carried by the reversible terminal can be removed after participating in DNA chain extension, so as to facilitate the next round of extension reaction.
[0237] These two aspects are at the heart of single-molecule high-throughput sequencing-by-synthesis (SMTS). details as follows:
[0238] 1) Preparation of DNA extension reaction system: Mix different reversible terminals in sequence with DNA template (known sequence information and known quality), Klenow (exo-) DNA polymerase, Klenow buffer, and stand at 30°C for 15 minutes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com