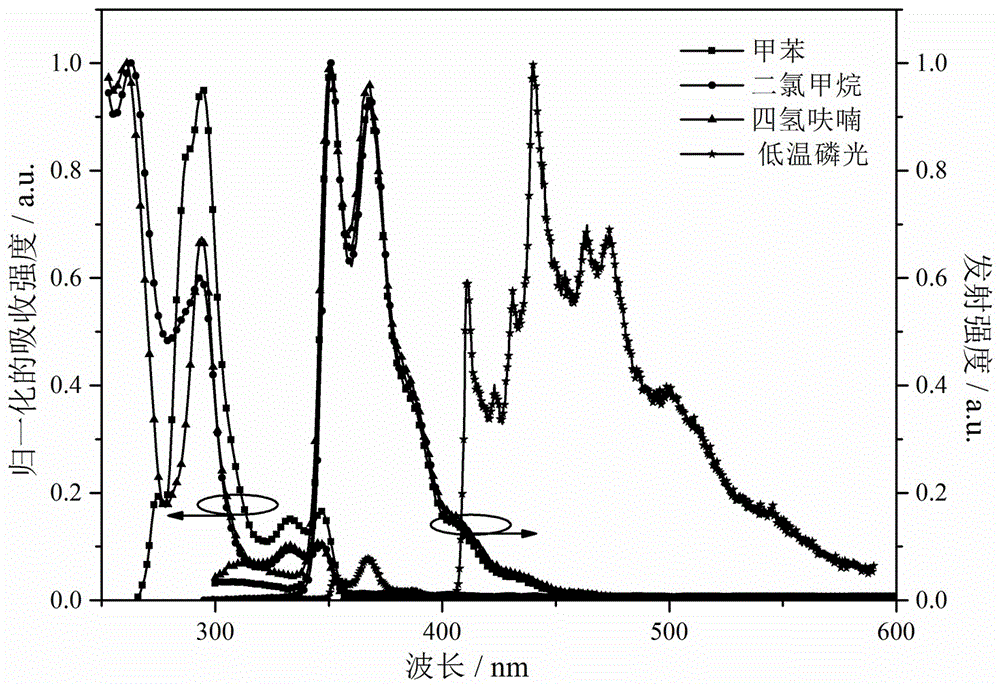
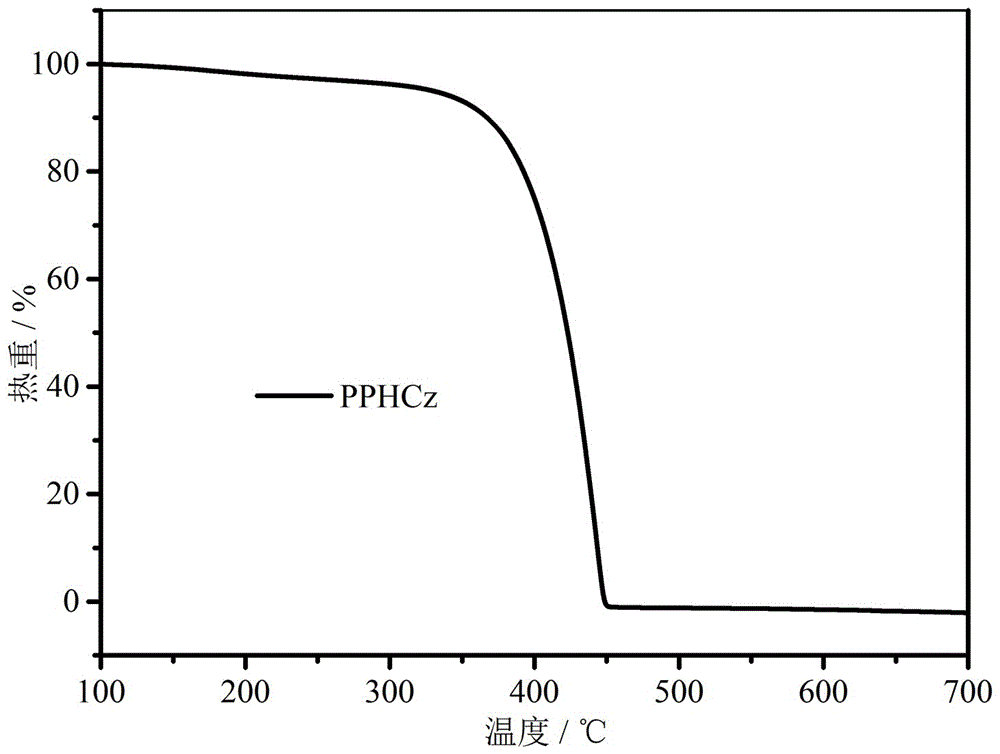
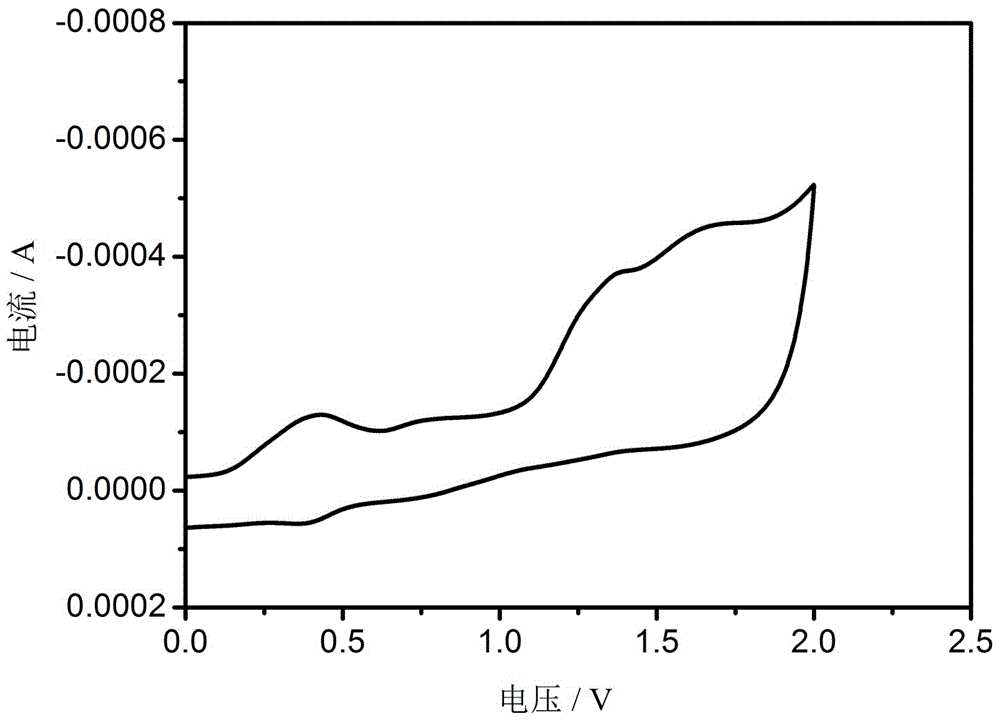
Bipolar blue phosphorescent host material based on carbazole and 1,2,4-triazole
A technology of blue phosphorescence and host materials, which is applied in the direction of luminescent materials, electric solid-state devices, semiconductor devices, etc., can solve the problem of not being able to meet the requirements of deep blue phosphorescent OLED devices, the triplet energy level is not very high, and reduce the triplet energy of the host material. Level and other issues, to achieve good double carrier transport characteristics, prevent energy return, and improve solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 2-cyanopyridine (5.21g, 50mmol), hydrazine hydrate (2.50g, 50mmol), and 25mL of ethanol were sequentially added to a 250mL two-necked round-bottomed flask, and reacted at a low temperature of 0°C for 8 hours to form a viscous light yellow paste. The excess ethanol was removed under vacuum at room temperature, the solid was washed with a small amount of ether, filtered, and dried under vacuum at room temperature for 3 hours to obtain white crystals of (2-pyridine)aminohydrazone.
[0034] (2-pyridine)aminohydrazone (4.08g, 30mmol), Na 2 CO 3 (3.15g, 30mmol), benzoyl chloride (4.20g, 30mmol), and 30mL tetrahydrofuran were added to a 250mL two-neck round bottom flask, reacted at room temperature for 6h, and filtered. The filtrate was refluxed in 30ml of ethylene glycol for 30min to dehydrate and ring-closed, filtered, dried in vacuo for 8h, and recrystallized from ethanol to obtain 2-(3-phenyl-1H-1,2,4-triazol-5-yl) Pyridine is white needle crystal, the yield is 92%.
[...
Embodiment 2
[0053] Carbazole (5.0g, 30mmol), 1,6-dibromobutane (31.9g, 150mmol), TBAB (1.0g, 3mmol), 40mL toluene, 50% KOH solution (25mL), were added to a 250mL two-necked round-bottomed flask in turn , reflux reaction under nitrogen protection for 12h. Cool to room temperature, add 50mL deionized water, extract with dichloromethane (50×3mL), wash the organic layer several times with deionized water, dry over anhydrous magnesium sulfate, filter, distill under reduced pressure, and separate and purify the crude product by column chromatography (Petroleum ether: dichloromethane = 8:1), 9-(6-bromobutyl)-9H-carbazole (L3) was obtained as white crystals with a yield of 80%.
[0054] 1 H NMR δ: 8.13(dt, 2H, J=7.8 Hz, 1.2 Hz), 7.48-7.47(m, 2H), 7.42(d, 2H, J=8.4 Hz), 7.26-7.24m, 2H), 4.35( d, 2H, J =7.2 Hz), 3.36(d, 1H, J =7.2 Hz), 1.96-1.80 (m, 1H), 1.52-1.39(m, 1H).
[0055] 2-(3-Phenyl-1H-1,2,4-triazol-5-yl)pyridine (2.21g, 10mmol), 9-(6-bromobutyl)-9H-carbazole (3.61g, 12mmol ), TBAB (0...
Embodiment 3
[0062] Carbazole (5.0g, 30mmol), 1,6-dibromoethane (27.8g, 150mmol), TBAB (1.0g, 3mmol), 40mL toluene, 50% KOH solution (25mL), were added to a 250mL two-necked round-bottomed flask in sequence , reflux reaction under nitrogen protection for 12h. Cool to room temperature, add 50mL deionized water, extract with dichloromethane (50×3mL), wash the organic layer several times with deionized water, dry over anhydrous magnesium sulfate, filter, distill under reduced pressure, and separate and purify the crude product by column chromatography (Petroleum ether: dichloromethane = 8:1), 9-(6-bromoethyl)-9H-carbazole was obtained as white crystals with a yield of 85%.
[0063] 2-(3-Phenyl-1H-1,2,4-triazol-5-yl)pyridine (2.21g, 10mmol), 9-(6-bromoethyl)-9H-carbazole (2.22g, 12mmol ), TBAB (0.32g, 1mmol), 30mL toluene, 50% KOH solution (15mL), were successively added to a 250mL two-necked round bottom flask, protected by nitrogen, stirred at room temperature for 30min, and heated to reflu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum brightness | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
| Maximum power efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com