Application of azalomycin F to preparation of medicines for treating atopic dermatitis
A technology for atopic dermatitis and azamycin, applied in the application field of azamycin F in the preparation of drugs for treating atopic dermatitis, can solve the problems of lack of curative drugs and complicated pathogenesis, and achieve significant anti-atopic dermatitis. Effects on atopic dermatitis effects, good applications and development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1.1 Experimental animals and reagents
[0022] Experimental animals: 72 clean-grade healthy female Kunming mice, weighing 18-22 g, were purchased from the Experimental Animal Center of Nanchang University.
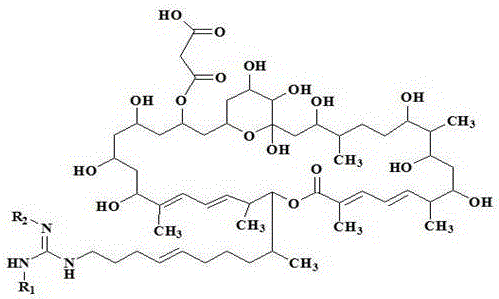
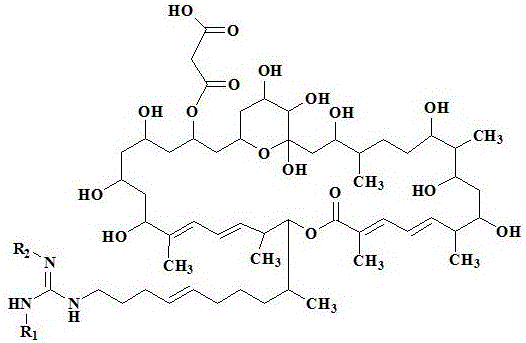
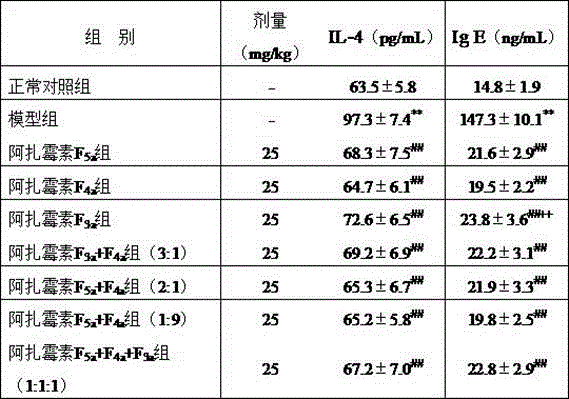
[0023] Reagent: Azamycin F 5a , Azamycin F 4a and azamycin F 3a Streptomyces Streptomyces hygroscopicus var .azalomyceticus , according to literature [2] Methods: The strains were fermented, and the fermentation products were prepared by solvent extraction, silica gel column chromatography and semi-preparative HPLC. The purity was 98.2%, 97.8% and 97.2%, respectively, and prepared with 0.15% CMC-Na aqueous solution before the experiment; 2, 4 - Dinitrofluorobenzene (DNFB) was purchased from Shanghai Chemical Reagent Company; mouse IL-4 and IgE ELISA kits were purchased from Invitrogen Company.
[0024] Establishment of atopic dermatitis model mice
[0025] 72 Kunming mice were randomly divided into model group, normal control group, azamycin F 5a group,...
Embodiment 2
[0058] 2.1 Experimental animals and reagents
[0059] Experimental animals: 91 clean-grade healthy female Kunming mice, weighing 18-22 g, were purchased from the Experimental Animal Center of Nanchang University.
[0060] Reagent: Azamycin F pure fractions A, B, C and D produced by Streptomyces Streptomyces hygroscopicus var .azalomyceticus , according to literature [2] The method is to carry out strain fermentation, and the fermentation product is obtained by solvent extraction and silica gel column chromatography; through HPLC analysis and determination, azamycin F in group A of azamycin F 3a , F 4a and F 5a The contents of Azamycin F in Group B were 61.7%, 33.5%, and 4.3%. 3a , F 4a and F 5a The contents of Azamycin F in group C were 13.5%, 73.2%, and 12.7%, respectively. 3a , F 4a and F 5a The contents of Azamycin F in Group D were 1.3%, 10.2%, and 87.8%. 4a and F 5a The contents of 2.9% and 96.7% were prepared with 0.15% CMC-Na aqueous solution before the ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com