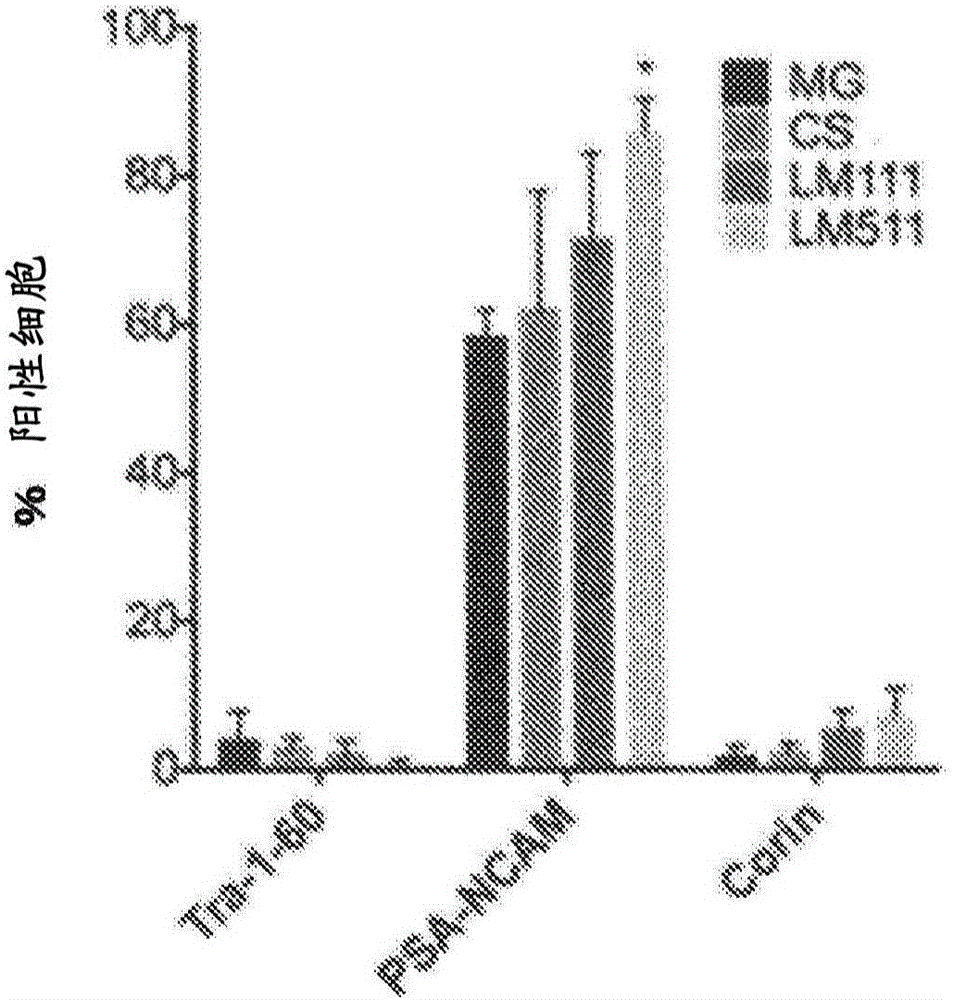
New method for inducing dopamine-producing neural precursor cells
A technology of dopamine nerve and precursor cells, applied in the direction of nervous system cells, artificially induced pluripotent cells, non-embryonic pluripotent stem cells, etc., can solve the problems of non-administration and achieve high implantation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] cells and culture
[0176] Human ES cells (KhES-1) were kindly provided by the Institute of Regenerative Medicine, Kyoto University (Suemori H, et al. Biochem Biophys Res Commun. 345:926-32, 2006). 404C2 and 836B3, which are human iPS cells, are obtained by introducing Oct3 / 4, Sox2, Klf4, L-MYC, LIN28, and p53shRNA into human fibroblasts using episomal vectors. Professor Yamanaka from Kyoto University et al. (Okita, et al, Nat Methods. 8:409-412, 2011).
[0177] ES cells and iPS cells were cultured based on the method described in Miyazaki T et al., Nat Commun. 3:1236, 2012. Briefly, 6-well plates coated with laminin 511E8 were used for culture.
[0178] The ES cells and iPS cells obtained in this way were dissociated with TrypLE CTS (Life Technologies), and each well was 4×10 4 Transfer each into an additionally prepared 6-well plate coated with laminin 511E8 (iMatrix-511, Nippi). After culturing for 4 days with the above-mentioned culture method, it was confirmed...
Embodiment 2
[0200] cell culture
[0201] ES cells (Kh-ES1) were dissociated using TrypLE CTS (Life Technologies), and all were transferred into an additionally prepared 6-well plate coated with laminin 511E8. 01 in minimal medium A (GMEM containing 8% KSR, 1 mM sodium pyruvate, 0.1 mM MEM non-essential amino acids and 0.1 mM 2-mercaptoethanol). One day after the initiation of culture (Day 1), the medium was replaced with minimal medium A containing 0.1 μM LDN193189, 0.5 μM A83-01, 2 μM purmorphamine, and 100 ng / mL FGF8. Three days after the initiation of culture (Day 3), the medium was replaced with minimal medium A containing 0.1 μM LDN193189, 0.5 μM A83-01, 2 μM purmorphamine, 100 ng / mL FGF8, and 3 μM CHIR99021. Seven days after the initiation of culture (day 7), the medium was replaced with minimal medium A containing 0.1 μM LDN193189 and 3 μM CHIR99021. On the 12th day (12 days after the start of culture), the medium was replaced with B27 supplement without vitamin A, 2 mM L-gluta...
Embodiment 3
[0209] cell culture
[0210] ES cells (Kh-ES1) were dissociated using TrypLE CTS (Life Technologies), and 4 × 10 5Each was transferred into a separately prepared 6-well plate coated with laminin 511E8, and cultured with StemFit medium (Ajinomoto) containing 10 μM Y-27632. After 4 days, the medium was replaced with the above minimal medium A containing 0.1 μM LDN193189, 0.5 μM A83-01 (day 0). One day after the initiation of culture (Day 1), the medium was replaced with minimal medium A containing 0.1 μM LDN193189, 0.5 μM A83-01, 2 μM purmorphamine, and 100 ng / mL FGF8. Three days after the initiation of culture (Day 3), the medium was replaced with minimal medium A containing 0.1 μM LDN193189, 0.5 μM A83-01, 2 μM purmorphamine, 100 ng / mL FGF8, and 3 μM CHIR99021. Seven days after the initiation of culture (day 7), the medium was replaced with minimal medium A containing 0.1 μM LDN193189 and 3 μM CHIR99021. On the 14th day (14 days after the start of culture), sort or replac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com