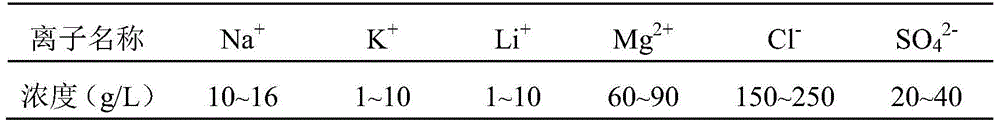Method for separating magnesium and lithium in salt lake brine and producing magnesium hydroxide and high-purity magnesium oxide
A technology of magnesium hydroxide and salt lake brine, applied in magnesium hydroxide, magnesium oxide and other directions, can solve the problems of abandonment of precious magnesium resources, underutilization of magnesium, waste of resources, etc., and achieves small loss rate, high purity, and separation process. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A. Salt lake brine solution A: [Mg 2+ ]=80g / L, [Li + ]=5g / L, take 250mL solution.
[0023] B. Weigh 66.7 g of NaOH and dissolve it in 250 mL of deionized water to obtain alkali solution B.
[0024] C. Pour the old brine solution A and alkali solution B into the colloid mill at the same time, and rotate at 1000r / min for 2 minutes to form Mg(OH) 2 Crystal nucleus, transfer the crystal nucleus solution to the reactor, stir and crystallize at 40°C for 10 hours, after the reaction, reduce the solution temperature to 20°C, and filter to obtain Mg(OH) 2 The filter cake was dried at 60°C for 8 hours to obtain solid Mg(OH) 2 Product, the molecular formula is: Mg(OH) 2 , Purity 99.0%
[0025] D. The Mg(OH) obtained in step C 2 In an air atmosphere, the solid is heated to 600°C at a heating rate of 5°C / min, kept for 6 hours, and calcined to obtain solid magnesium oxide. The molecular formula is MgO, and the purity is 99.0%.
[0026] E. Evaporate and concentrate the filtrate of step C at 40...
Embodiment 2
[0028] A. Salt lake brine solution A: [Mg 2+ ]=60g / L, [Li + ] = 1g / L, take 250mL solution.
[0029] B. Weigh 50g of NaOH and dissolve it in 250 mL of deionized water to obtain alkaline solution B.
[0030] C. Pour the old brine solution A and alkali solution B into the colloid mill at the same time, and rotate at 1000r / min for 2 minutes to form Mg(OH) 2 Crystal nucleus, transfer the crystal nucleus solution to the reactor, stir and crystallize at 40°C for 10 hours, after the reaction, reduce the solution temperature to 20°C, and filter to obtain Mg(OH) 2 The filter cake was dried at 60°C for 8 hours to obtain solid Mg(OH) 2 Product, the molecular formula is: Mg(OH) 2 , The purity is 99.2%.
[0031] D. The Mg(OH) obtained in step C 2 The solid is calcined in an air atmosphere, the heating rate is 2°C / min, the temperature is increased to 500°C, and the temperature is kept for 5 hours to obtain solid magnesium oxide. The molecular formula is MgO and the purity is 99.2%.
[0032] E. Evapor...
Embodiment 3
[0034] A. Salt lake brine solution A: [Mg 2+ ]=70g / L, [Li + ]=3g / L, take 250mL solution.
[0035] B. Weigh 58.3 g of NaOH and dissolve it in 250 mL of deionized water to obtain alkali solution B.
[0036] C. Pour the old brine solution A and alkali solution B into the colloid mill at the same time, and rotate at 3000r / min for 3 minutes to form Mg(OH) 2 Crystal nucleus, transfer the crystal nucleus solution to the reactor, stir and crystallize at 50℃ for 12 hours, after the reaction, reduce the temperature of the solution to 30℃, and filter to obtain Mg(OH) 2 The filter cake was dried at 70°C for 10 hours to obtain solid Mg(OH) 2 Product, the molecular formula is: Mg(OH) 2 , The purity is 99.0%.
[0037] D. The Mg(OH) obtained in step C 2 The solid is calcined in an air atmosphere, the heating rate is 7°C / min, the temperature is increased to 700°C, and the temperature is kept for 8 hours to obtain solid magnesium oxide. The molecular formula is MgO and the purity is 99.0%.
[0038] E. E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com